Oophorectomy At Hysterectomy After Age 40? A Practice That Does Not Withstand Scrutiny
As published in Vol 5, Number 5 (Dec, 1996) of Menopause Management, the journal of the North American Menopause Society, for Health Care Professionals.
Click here to watch the recent video interview of Dr. Cutler discussing her new book; Hormones and Your Health
*Please click here for more on pheromones and books from Athena Institute
By Winnifred B. Cutler (Athena Institute for Women's Wellness Research, Chester Springs, PA, USA)
Surgical removal of gonads (castration) alters the hormonal milieu in a number of different ways that may precipitate chronic health problems and increased costs. Moreover, regimens designed to replace estrogen alone overlook the many ovarian contributions to the life of a maturing woman.
- Introduction
- Incidence and Risk Factors
- Post Oophorectomy Sequellae
- Ovarian Contribution
- Alternatives to Prophylactic Oophorectomy
- Conclusions and References
Introduction
Surgical removal of gonads (castration) alters the hormonal milieu in a number of different ways that may precipitate chronic health problems and increased costs. Moreover, regimens designed to replace estrogen alone overlook the many ovarian contributions to the life of a maturing woman.
Before research papers began revealing many negative effects of such practice, healthy ovaries were routinely removed at hysterectomy in North America in the 70s and 80s. Today, research available through a simple Medline search leads me to seriously question the rationality and ethical propriety (in the post-forty- year old woman) of routinely removing healthy ovaries during a hysterectomy in the 99.9% of cases with a less than 1% risk of ovarian cancer.
Nevertheless, physicians reading the 1994 journal Gynecologic Oncology1 would see this sentence: "If access to the pelvic organs is possible, women undergoing non-gynecologic surgeries at the age of 40 or older should be encouraged to consider a prophylactic oophorectomy." However, further correspondence in another journal seriously challenged this recommendation.2
I suggest that the practice of prophylactic castration - surgical removal of healthy ovaries based on future possibility of disease - cannot withstand scrutiny. The possible devastation of ovarian cancer must be carefully balanced against the devastation of the removal of healthy organs from those women who are not likely to be at risk for ovarian cancer.
Incidence and Risk Factors
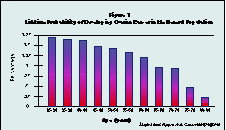
The existence of ovarian cancer, or its prevention, appear to be the only rational reasons for considering ovariectomy during hysterectomy. Actual lifetime risk of ovarian cancer declines with age (see figure 1.)The lifetime probability of ovarian cancer in the general population by age group is highly dependent on family history (see figure 2). A woman with no family history of ovarian cancer has about a 1% lifetime risk of contracting the disease.
(Click image to see Figure 1 full-size)
Hysterectomy, itself, further reduces the relative risk of subsequently developing ovarian cancer. For example, in a prospective study of 122,000 nurses with 12 years of follow up hysterectomy was associated with a reduced risk of ovarian cancer: Risk Ratio (RR) = .67.3 According to Prazzini, who compared 943 cases of ovarian cancer with 2503 controls, the (confirmed) reduction in risk after hysterectomy is time dependent, reaching a RR of .5 after 15 years post-hysterectomy. 4
(Click image to see Figure 2 full-size)

To ask the question, "Does ovariectomy prevent ovarian cancer?" for a patient with no family history of ovarian cancer is misleading. The answer is "probably, yes" but the price is too high for a woman with a less than 1% relative risk of ovarian cancer.
After reviewing familial risk factors and the role of prophylactic oophorectomy in cancer prevention Nguyen and colleagues,5 as well as Herbst,6 concluded that it may be warranted only for women with family ovarian cancer syndrome. This is an autosomal dominant inheritance disease that occurs in less than 1% of all ovarian cancer cases (Herbst, 1994), and produces a 50% lifetime risk of developing ovarian cancer. In contrast, the presence of one, two, or three family members with ovarian cancer increases the lifetime risk from 1% among all women, to between 5% and 7% depending on the number of affected relatives (Nguyen, 94) (see Fig. 2).
Assuming an annual incidence of 22,000 cases of ovarian cancer in the United States and combining that information with the percentage of these cancers that occur in women over the age of 40 led the authors to conclude that 1000 women might be spared ovarian cancer by routine castration of all women over 40 during hysterectomy.
Following this logic, I estimate that 399,000 unnecessary castrations would have to be performed during a hysterectomy for benign pathology in order to prevent 1000 cases of ovarian cancer in the U.S. per year. Since the practice of hysterectomy reduces by 30 to 50% the likelihood of developing subsequent ovarian cancer the overall risk of this cancer after hysterectomy approaches half the lifetime risk of 1%. Since the risk of ovarian cancer also declines with age after 50, the risk of ovarian cancer in a woman without family history approaches one-quarter one percent. Thus over 99.75% of the "prophylactic ovariectomy" procedures would be unnecessary. To use surgery in order to spare 1000 women at risk, 400,000 women would need to be castrated.
Post Oophorectomy Sequellae
Unfortunately, substantial noncompliance with HRT prescriptions, as well as the incorrect identification of adequate hormonal replacement therapy produce serious sequellae after prophylactic oophorectomy. If women will not take the prescribed hormone regimens, they will show excessive rates of heart disease and osteoporosis while only moderately reducing their very low risk of a rare disease.
Alterations in sexuality are inevitable as a result of reductions of both estrogenic and androgenic hormones. Adequate vaginal lubrication requires estrogen and adequate libido requires sufficient androgen circulation; deficits are inevitable if these hormones are reduced.
Research in subhuman primates and subsequently in humans revealed that the endogenous opiates fall precipitously after surgical disruption of the estrogen/progesterone cycle.7 Ovariectomy may compromise a woman's sexual attractiveness in part due to obliteration of her axillary pheromonal secretions. In Rhesus monkeys, castration eliminates the production of sexual attractants (pheromones) and leads to an immediate loss of capacity to attract the male. Only when sex steroids were replaced appropriately or when pheromonal secretions were applied vaginally, did the monkey regain sexual attention from the male.8 Human pheromone research is in its infancy and only limited research is available to address the potential loss of sexual attractiveness a castrated woman may suffer.
Ovarian Contribution
The classic studies of human ovarian cross sections clearly demonstrated an inevitable change from infancy to old age. After achieving peak size, average ovarian mass declines in women between the ages of 30-39. However the decline is accompanied by concomitantly increasing quantity of stromal tissue. The postmenopausal ovary is rich in stromal tissue (which provides a rich source of androgens),9 often reaching higher levels than during the fertile years. Healthy older women (60-98 years old) showed active hormonal secretion: they achieved plasma levels of estradiol averaging 16 pg/ml; testosterone, 270 pg/ml; and DHEAS, 360 ng/ml. 10 (Age-related decline in DHEAS, is well documented.11 and castration accelerates that decline by 50%.12 It remains to be clarified whether the castration-induced diminution of DHEAS reflects an adrenal dependence on the ovary, some aspect of the ovarian contribution itself, or both. However, since DHEAS is a precursor hormone to other sex steroids, its role is magnified.
The potential health benefits of circulating DHEAS have recently achieved considerable scientific, as well as media, attention. Its role in health and well being became apparent by the mid 1990's. A Medline search for this subject will reveal numerous recently published studies on DHEAS. DHEAS replacement therapy has not yet been adequately studied but the concept is beginning to percolate into the literature. Just a brief smattering of studies cited below reveal the potential health values of retained ovaries (which support higher natural levels of DHEA/S):
Secretion of DHEA is much lower in women with breast cancer and several studies have shown subnormal human plasma levels of both DHEAS and DHEA in advanced breast cancer patients. DHEA inhibits three processes that contribute to tumor development:
1) Metabolic activation of a carcinogen through the activation of mixed function oxidases
2) Tumor promoter stimulation of cell proliferation
3) Tumor promoter of O2- formation.13
In 1994 Mason and colleagues showed that breast cancer survival is significantly related to higher plasma DHEA levels and tumor steroid receptor status.14 DHEA levels below the average of the group were associated with significantly decreased survival in women. Particularly apparent in postmenopausal receptor positive tumors, the association was most powerful in those who were axillary node negative.14
High levels of DHEAS in men were associated with reduced fatality from cardiovascular disease,15 whereas oral DHEA to postmenopausal women increased lipid risk factors.16 Examination of the records of 942 post-menopausal women revealed that higher baseline DHEAS levels associated with several major CVD risk factors 12 years later; but such levels were not associated with mortality risk in these women.17 Though excessive doses may be dangerous to cardiovascular health in women, these apparently disparate results render the potential roles of DHEAS in cardiovascular health a ripe subject for future investigation.
Castration cuts the already diminshing levels of DHEAS in aging women in half
The relationships among calcium absorption, serum DHEA and vertebral mineral density in post-menopausal woman were investigated as early as 1985. Malabsorption of calcium was a significant risk factor for osteoporosis, and low DHEA levels appear to represent an additional independent risk factor.18 More recently, roles for DHEA and DHEAS have been suggested in the regulation of human osteoblast function as shown in cultures of cells responsible for the synthesis and mineralization of bone.19
DHEAS and DHEA affected brain cells beneficially, enhancing nerve cell cultures of mouse brain.20 Patients with Alzheimer's showed an age-related decrement in DHEA, about 40% lower than their age matched non-diseased contemporaries.21
The developing and proliferating field of DHEA and DHEAS research is sizeable enough to permit evaluation within the limitations of current knowledge. What is unambiguous remains--castration cuts the already diminishing levels of DHEAS in aging women in half.12 Though the physiological effects of unreplaced DHEAS are as yet unknown there is a rational basis for DHEA replacement therapy in postmenopausal women. Studies of DHEA replacement therapy have already begun to appear, and eventually optimal levels and safety issues will be resolved.22
Alternatives to Prophylactic Oophorectomy
In the presence of ovarian mass, recent improvements in the methodology to detect ovarian cancer lessen justification for the removal of healthy ovaries. In some hands improved techniques can profoundly increase the positive predictive value to 97%.5,23,24 According to Weiner et al,24 transvaginal color flow imaging with pulsed doppler ultrasound can detect even early stage ovarian cancers because of the dramatic increase in blood flow to the region of early stage ovarian cancers. While these studies have only recently begun to appear, they may be of benefit in the future.There is no current screening method proved to reduce mortality when there is no mass.
The physician's role in informing the patient must be to:1) Define the problem (no effective screen available)2) Describe the truly low risk of ovarian cancer3) Elucidate the health hazards of castration.For irrational fear of cancer physicians should recommend help from professional counselors, not unnecessary castration.Better diagnostic techniques now available can spare women from unnecessary surgery in the presence of ovarian mass.
Conclusions and References
Conclusion
Better diagnostic techniques now available can spare women from unnecessary surgery in the presence of ovarian mass. Physicians should utilize continuing education courses to learn these detections methods as the new standard of care. The focus should be on developing better detection techniques rather than on convincing women to accept unnecessary castration in order to prevent a less than one-half percent risk of ovarian cancer.
As consumers of health care, women who are informed will understandably demand their rights to make rational decisions. Women are learning about castration's effects:
the reduced well being, the time for recovery or healing from pelvic surgery before they can return to full functioning, and the associated loss of time, energy, and money. The increasing multitude of functions from ovaries continues to be exposed as research studies progress. As these healthcare consumers recognize the hormonal contribution of even aging ovaries, and the sexual sequelae following their loss, they are likely to say "no". Most importantly, as women discover how low the ovarian cancer risk is, for the 99.9% without the high risk autosomal dominant syndrome, they can be expected to react strenuously against the suggestion they be castrated.
As the true costs of "prophylactic oophorectomy" become more widely recognized by medical insurers and the informed consumer, the reduction in such a practice seems inevitable. Providers of financial resources for surgery can be expected to deny funding as they recognize both direct and subsequent costs imposed by pelvic surgery.25 The potential increase in cardiovascular disease, osteoporosis, sexual dysfunction, and psychopathology resulting from prophylactic oophorectomy increases the health cost burden. Inevitably, managed care organizations, as well as those who self-pay for medical service, can be expected to withhold funds for "prophylactic" surgeries.
Medical professionals apprised of the proliferation of new research should welcome this reduction in surgery. The medical establishment is already reeling from the dramatic changes in financial structure imposed by managed care. A focus on better detection of pathology and understanding the elegant symphony of a woman's intact, albeit aging body can result in improved medical care.
References
1) -Averette HE, Nguyen HN (1994) The role of prophylactic oophorectomy in cancer prevention. Gynecologic Oncology, 55: S38-S41
2) -Utian WH, GoldfarbJ. Ovarian management during radical hysterectomy in the premenopausal patient. Obstet Gynecol 1993;82(6):1042
3) -Hankinson SE, Hunter DJ, Colditz GA, Willett WC, Stampfer MJ, Rosner B, Hennekens CH, Speizer FE (1993) Tubal ligation, hysterectomy, and risk of ovarian cancer: A prospective study. JAMA, 270, 23: 2813-2818
4) -Prazzini F, Negri E, La Vecchia C, Luchini L, Mezzopane R. (1993) Hysterectomy, oophorectomy, and subsequent ovarian cancer. Obstet Gynecol 81:363-366
5) -Nguyen HN, Averette HE, Janicek M. (1994) Ovarian carcinoma: A review of the significance of familial risk factors and the role of prophylactic oophorectomy in cancer prevention. Cancer 74, 2:545-555
6) -Herbst AL. (1994) The epidemiology of ovarian carcinoma and the current status of tumor markers to detect disease. Am J ObstetGynecol 170, 4:1099-1107
7) -Cutler WB, (1990) Hysterectomy Before and After HarperCollins, NY.pp 40-41 and 256-257
8) -Cutler WB (1997) Pheromonal Modulation of Brain and Behavior, an invited chapter, under review in Hormonal Modulation of Brain and Behavior, American Psychiatric Press.
9) -Cutler WB and Garcia CR. (1984) The Medical Management of the Menopause and Premenopause: Their Endocrinologic Basis. Lippincott Co., Philadelphia, PA.
10) -Eggert-Kruse W, Kruse W, Rohr G, Muller S & others (1994) Hormone profile of elderly women and potential modifiers. Geburtshilfe Frauenheilkd 54, 6:321-31
11) -Carlstrom K, Brody S, Lunell NO, Lagrelius A, Mollerstrom G, Pousette A, Rannevik G, Stege R, von Schoultz B (1988) Dehydroepiandrosterone in serum: Differences related to age and sex. Maturitas 10:297-306
12) -Cumming DC, Rebar RW, Hopper BR, Yen SSC (1982) Evidence or an influence of the ovary on circulating dehydroepiandrosterone sulfate levels. J of Clinical Endocrinology & Metabolism 54, 5:1069-1071
13) -Schwartz AG, Whitcomb JM, Nyce JW, Lewbart ML, Pashko LL. (1988) Dehydroepiandrosterone and structural analogs: A new class of cancer chemopreventive agents. Advances in Cancer Research 51:391-425
14) -Mason BH, Holdaway IM, Skinner SJ, Kay RG (1994) The relationship of urinary and plasma androgens to steroid receptors and menopausal status in breast cancer patients and their influence on survival. Breast Cancer Res Treat 32, 2:203-12
15) -Barrett-Connor E, Khaw K-t, Yen S (1986) A prospective study of dehydroepiandrosterone sulfate, mortality, and cardiovascular disease. New England J of Med, 315:1519-1524
16) -Mortola JF and Yen SC (1990) The effects of oral dehydroepiandrosterone on endocrine-metabolic parameters in postmenopausal women. J Clin Endocrinol Metab 696-704.
17) -Barrett-Connor E, Goodman-Gruen D (1995) Dehydroepiandrosterone sulfate does not predict cardiovascular death in postmenopausal women. The Rancho Bernardo Study. Dept of Family & Preventive Medicine 91, 6:1757-60
18) -Nordin BEC, Robertson A, Seamark RF, Bridges A, Philcox JC, Need AG, Horowitz M, Morris HA, Deam S. (1985) The relation between calcium absorption, serum dehydroepiandrosterone, and vertebral mineral density in postmenopausal women. J of Clin Endocrinology & Metabolism 60:651-657
19) -Bodine PV, Riggs BL, Spelsberg TC (1995) Regulation of c-fos expression and TGF-beta production by gonadal and adrenal androgens in normal human osteoblastic cells. J Steroid Biochem Mol Biol 52, 2:149-58
20) -Bologna I, Sharma J, Roberts E (1987) Dehydroepiandrosterone and its sulfated derivative reduce neuronal death and enhance astrocyctic differentiation in brain cell cultures. J of Neuroscience Research 17:225-234
21) -Sunderland T, Merril CR, Harrington MG, Lawlor BA, Molchan SE, Martinez R, Murphy DL. (1989) Reduced plasma dehydroepiandrosterone concentrations in alzheimer's disease. Lancet pg 570
22) -Casson P, Faquin L, Stentz F, Straughn A, Andersen R, Abraham G, Buster J (1995) Replacement of dehydroepiandrosterone enhances T-lymphocyte insulin binding in postmenopausal women. Fertil/Steril 63:1027-1037
23) -Goldstein S, Suramanyam B, Snyder J, Beller U, Raghavendra BN, Beckman EM(1989) The postmenopausal cystic adnexal mass: The potential role of ultrasound in conservative management. Obstet Gynecol 73:8-10
24)-Weiner Z, Thaler I, Beck D, Rotten S, Deutsch M, Brandes J. (1992) Differentiating malignant from benign ovarian tumors with transvaginal colorflow imaging. Obstet Gynecol 79:159-162
25) -Brumstead JR, Blackman JA, Badger GJ, Riddick DH (1996) Hysteroscopy versus hysterectomy for the treatment of abnormal uterine bleeding: A comparison of cost. Fertility and Sterility 65:310-316
ACKNOWLEDGMENTS
I thank Norma McCoy, Ph.D., Professor of Psychology, San Francisco State University, and Brooke Wollenberg at the Athena Institute for help in the research and editing preparation of the manuscript and both Elizabeth Genovese Stone, M.D. Medical Director of Athena Institute and Millicent Zacher, D.O., Acting Director of Division of Reproductive Endocrinology and Infertility, Dept of Ob/Gyn, Albert Einstein Medical Center, Philadelphia for significant comments on medical perspectives.
Copyright (c) 1996 Athena Institute. All rights reserved.
Reproduction in whole or in part in any form or medium without express written permission of Athena Institute is prohibited
" My research has consistently focused on what behavior a woman can engage in to increase her power, well-being, and vitality."
---Winnifred B. Cutler, Ph.D.
A portion of the profits from our book and pheromone sales helps to fund Athena's on-going research.