Sexual behavior and steroid levels among gynecologically mature premenopausal women.

Winnifred B. Cutler, Ph.D. (click for bio), Celso-Ramon Garcia, M.D., George R. Huggins, M.D., George Preti, Ph.D.
Fertility and Sterility, Vol. 45, No. 4, April 1986.
© Copyright 1986. The American Fertility Society
Comments from Dr. Cutler, 2011 --
"The paper posted below was published in 1986, and introduced some of my research that remains as relevant to the body of health care knowledge today particularly because women are being (incorrectly) told that their natural femininity, reflected in robust and high estrogen levels, is dangerous for their breasts. Twenty-five years later, the conclusion of this paper is still an essential message to women -- that a definite relationship exists between our sexual behavior and our fertility, estrogen levels, reproductive endocrinology, and overall health. And that women who experience a regular stable, ongoing sexual life with men appear to be protected against the deficient levels of estrogen that put our bones and other physiologic systems at risk of early aging.
Since this paper I have published studies and written books on women's health; many if not all refer back to the finding that women who engage in weekly, regular sexual behavior (like a paycheck never missing a non-menstruating week) have more optimal fertile-type menstrual cycles compared to women who have sporadic sexual behavior and are significantly more likely to experience sub-fertile type menstrual cycles and subnormal levels of estrogen.
And the impact of sexual behavior reaches beyond a woman's reproductive years, because this 'weekly' pattern delays the onset of menopause and in doing that, extends the lifespan. As stated in this paper 'Thus, a pattern emerges that suggests that women whose heterosexual behavior is maintained at a minimum frequency of once per non-menstruating week demonstrate endocrine-related effects, as reflected in their cycle lengths, estrogen levels, and BBT (basal body temperature) patterns.' Women who have higher levels of estrogen before menopause appear to be more protected after menopause from pervasive age-related diseases such as osteoporosis and atherosclerosis."
Winnifred Cutler, Ph.D.
Founder, Athena Institute for Women's Wellness, Inc.
Abstract:
-- Fertil Steril 45:496, 1986
Intro:
A variety of relationships between the sexual behavior of women and multifaceted aspects of their potential for fertility have recently been reported. 1-8 Heterosexual behavior is essential for the gamete interactions of insemination and impregnation. However, recently published studies 1-9 suggest that heterosexual behaviors influence the reproductive endocrine system. These behavioral-physiologic studies were performed with the use of three population bases: infertile women, gynecologically mature healthy young women, and healthy perimenopausal women.
The length of a woman’s menstrual cycle has long been known to reflect the underlying endocrine milieu, which includes the potential for fertility. Vollman 10-12 as well as Treloar et al., 13 have shown, through their extensive prospective studies, that women whose menstrual cycles approach the 29-day span are the ones with the highest likelihood of fertile cycles. Women whose cycles become increasingly longer or shorter have a proportionately increasing incidence of sub-fertile menstrual cycle patterns. These abnormalities are present in a variety of ways: absence of ovulation, deficient lengths of the postovulatory phase, inadequate progesterone (P) levels, and excessively long follicular phases. 3 We have recently demonstrated relationships between (1) age at first coitus and subsequent infertility and (2) approximately 29-day cycles and weekly sexual behavior in several different populations collected during different years.
Among a population of infertile women, a delayed age of first coitus appeared, compared with age at first coitus in fertile women. 1,2 In addition, one study suggested that optimum fertility is obtained if first coitus occurs within 7 years after the onset of menarche. 1
Our first prospective, double-blind study at the University of Pennsylvania was published in 1979 (click for more).4 It showed that women who had regular weekly heterosexual activity had menstrual cycles of about 29 days, whereas women who either were celibate or who engaged in sporadic (less than weekly) coital behavior tended to have a high frequency of aberrant cycle lengths (less than 26 or more than 33 days).4 This initial study4 reported the phenomenon in women pre-selected on the basis of their gynecological maturity (menstruating at least 7 years 13), nonuse of oral contraceptives or intrauterine devices, and nulliparity. Subsequent reports3 showed that, in a clinical population of infertility patients (C.-R.G.), shortened hyperthermic basal body temperature (BBT) phase lengths and sporadic sexual activity in the luteal phase were associated.3
We recently reported5 a replication and expansion of the earlier findings in a presumably healthy college-student sample. We showed that women with regular weekly coital activity had the highest incidence (90%) of presumptively fertile BBT rhythms: sporadically active women had the next highest incidence (55%); and celibate women revealed the lowest incidence (44%) of presumptively fertile-type BBTs.
Following similar protocols, we examined the sexual behavior of perimenopausal women in a prospective double-blind fashion. Hot flashes and sexual behavior were shown to be inversely related to each other; and perimenopausal women who were coitally active at least once weekly showed higher levels of estrogen than their age-matched peers who were less sexually active.6 In that study, although higher levels of estrogen were found in the weekly group than in the less than weekly, no difference in testosterone (T) levels was found between the groups.
It is especially noteworthy that in each of the previous studies, the behavioral dichotomy (weekly versus less than weekly) is useful in revealing an association between sexual behavior and aspects of fertile endocrine milieu; the total quantity of behavior appears to be an irrelevant marker for fertile patterns of menstrual cycling. For example, a subsequent report7 of the perimenopausal sample showed that hormone levels were not significantly associated with the total frequency of sexual activity (measured by the total days of coital encounter throughout a 10-week span). In addition, there is a significant association between high frequencies of sporadic behavior and a high likelihood of aberrant cycle length among sporadically active young women.8
Thus, a pattern emerges that suggests that women whose heterosexual behavior is maintained at a minimum frequency of once per non-menstruating week demonstrate endocrine-related effects, as reflected in their cycle lengths, estrogen levels, and BBT patterns. However, these effects are not associated with total quantity of behavior or with masturbation. Although weekly sexual behavior does predict a fertile-type endocrine milieu, the reverse has not been shown. That is, a 29.5+ 3-day cycle length, a fertile-type BBT pattern, or a higher estrogen level will not predict weekly sexual behavior. Thus, stability in sexual behavior is associated with stability in endocrine rhythm, but endocrine stability does not predict sexual behavior.
The study reported here describes the relationship between sex steroid levels (i.e., estradiol [E2], P, and T) and frequency of sexual behavior among young, healthy women.
MATERIALS AND METHODS
Data from 27 subjects who completed this study are presented in Table 1. These subjects were recruited locally from among students and office workers and were paid for their participation. All met the following criteria: gynecologic maturity, nulliparity, not currently (nor within the last 3 months) using oral contraceptives or an IUD, no marijuana or other "pleasure" drugs, and a willingness to make a daily entry of BBT, sexual behavior, and menstrual occurrence.
All subjects who began the experiment reporting themselves to be aberrant cyclers (n= 28) agreed to undergo a complete history and pelvic examination, which was performed by one of us (G.R.H.). This screening process for possible pathologic factors that might influence the length of the menstrual cycle failed to find any. Each subject also agreed to provide blood samples for steroid analysis during 3 days of 1 week in the luteal phase of the last menstrual cycle studied. Forty-four women were enrolled originally. Of the 28 aberrant cyclers, 9 dropped out before supplying blood samples and 5 supplied samples too late in the study for an evaluation of where within a BBT cycle they had occurred. Of the 16 self-reported approximately 29-day cyclers, 2 dropped out before supplying blood and 1 supplied blood too late for evaluation. The study began September 1, 1983 and was scheduled to be completed by December 15, 1983. Human studies approvals were obtained before the study began.
Table 1. The Subjects and Their Data
Sexual
Behavior |
Hormones |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Subject | BBT | Cycle length | Sampling day | Heterosexual total |
Age | Heterosexual categorya |
E2 pg/ml |
T ng/ml |
P ng/ml |
|
| 1 | 1 | 31 | 9,8,4 | 2 | 21 | S | 99 | 0.81 | 6.41 | |
| 2 | + | 25 | 5,1,4 | 3 | 21 | S | 180 | 0.55 | 7.56 | |
| 3 | 1 | 28 | 9,8,7 | 7 | 21 | S | 126 | 0.25 | 18.05 | |
| 4 | 1 | 25 | 2,1 | 28 | 26 | W | 81 | 0.69 | 1.17 | |
| 5 | 1 | 22 | 19,21 | 3 | 20 | S | 4 | 16.96b | 0.77 | |
| 6 | 2 | 24 | 2,6 | 5 | 18 | S | 7 | 0.41 | 1.31 | |
| 7 | 1 | 28 | 4,7 | 5 | 37 | S | 91 | 0.60 | 12.02 | |
| 8 | + | 28 | 13,8,7 | 18 | 24 | S | 66 | 1.23 | 14.09 | |
| 9 | + | 27 | 5,8,9 | 5 | 27 | S | 82 | 0.48 | 10.98 | |
| 10 | + | 27 | 8,6 | 0 | 29 | N | 83 | 0.35 | 12.08 | |
| 11 | + | 28 | 10 | 25 | 27 | W | 336 | 0.07 | 15.05 | |
| 12 | 1 | 29 | 8,6,4 | 18 | 22 | S | 78 | 1.33 | 11.11 | |
| 13 | 1 | 26 | 3,2,1 | 0 | 19 | N | 46 | 0.38 | 1.62 | |
| 14 | + | 28 | 11,10,9 | 43 | 32 | W | 80 | 0.78 | 5.08 | |
| 15 | + | 27 | 9,7,6 | 0 | 18 | N | 61 | 0.47 | 8.95 | |
| 16 | 1 | 29 | 11,5,4 | 39 | 19 | W | 84 | 0.37 | 3.60 | |
| 17 | 54 | (19,21,24) | 4 | 21 | S | 37 | 1.29 | 5.50 | ||
| 18 | + | 35 | 15,13,11 | 5 | 21 | S | 27 | 0.83 | 2.52 | |
| 19 | + | 40 | 27,26,25 | 32 | 27 | W | 158 | 0.91 | 0.49 | |
| 20 | 1 | 27 | 3 | 42 | 30 | W | 111 | 1.82 | 5.91 | |
| 21 | 1 | 41 | 21,20,17 | 8 | 19 | S | 37 | 0.96 | 0.71 | |
| 22 | + | 26 | 4,3,2 | 0 | 19 | N | 52 | 1.10 | 2.73 | |
| 23 | + | 25 | 4,3,2 | 0 | 20 | N | 67 | 0.42 | 6.03 | |
| 24 | + | 39 | 20,19,18 | 33 | 20 | W | 34 | 1.01 | 0.44 | |
| 25 | + | 29 | 11,10,9 | 26 | 20 | S | 139 | 1.09 | 6.95 | |
| 26 | 1 | 27 | 23,21,20 | 4 | 21 | S | 21 | 2.98 | 1.01 | |
| 27 | 1 | 31 | 18,9,8,7 | 4 | 19 | S | 14 | 0.72 | 1.85 | |
a S, sporadic; W, weekly; N, never.
b This figure not included in normative data analysis.
EXPERIMENTAL PROTOCOL
The study was double-blind. Each subject was provided with the BBT charts, BBT thermometers, and a calendar card to record sexual behavior. Instructions for their use were given by a technician who was blind to the purpose of the study. The average age of the subjects was 22.9 years of age (Table 1). Subjects were told that the research was designed to increase our understanding of the reproductive endocrine system of women.
Subjects came into the laboratory three times weekly for the 14 weeks of the study, exchanging data cards and BBT charts each month as well as having their pulse and blood pressure measured. The latter was designed to dilute the subjects’ perception of the sexual content focus of the study. Other details of their laboratory experience not directly relevant to this report appear in another manuscript, which, as of this writing, is unpublished but has been submitted for publication.14 At each visit, an emphasis was placed on the importance of the subject’s contribution to knowledge and, therefore, how very relevant it was that she not back-fill on her data cards but rather prospectively record events each day. In the third cycle, plasma samples were drawn at the most likely midluteal phase time of the cycle. The time of sampling was based on our review of the current BBT chart and previous two cycle lengths. In several cases, illness or an unexpected change of plans by the subject prevented collection of three plasma samples.
Table 1 shows each subject’s plasma sampling schedule in reverse days to next menses for all but the one subject (17) who did not experience another menses before the study ended. For her, actual cycle days since the last menses are indicated and brackets placed around the numbers. At the conclusion of the study, subjects were asked to fill out a questionnaire about the rigor with which they kept track of the data required from them (see ”Validation of Accuracy”).
PLASMA SAMPLES/RADIOIMMUNOASSAY
Blood samples were withdrawn into heparinized vacutainers and separated with the use of centrifugation (2500 x g for 30 minutes). The plasma was collected and kept frozen at -180C until assayed. Thawed plasma aliquots (1 ml) were extracted with 10 ml of acid-washed, peroxide-free diethyl ether, followed by 10ml of light petroleum spirit extract (380 to 560C). The combined extract was blown dry in a warm sand bath by a gentle stream of dry nitrogen and analyzed for E2, T, and P according to the method of Katz et al.15 The mean value for each steroid (three samples) for each subject is shown in Table 1. One subject (5) showed extremely high T values, suggestive of endocrine pathologic factors. Because of the mobility of the population and the 2-year delay to data analyses of the frozen samples, attempts to locate the subject with the elevated T values have been unsuccessful.
BASAL BODY TEMPERATURE AND SEXUAL BEHAVIORAL SCORING
Each basal body chart was scored in double-blind fashion by a research technician and checked by one of us (G.P.). The chart was categorized as presumed normal ovulatory (+), anovulatory (1), or presumed ovulatory with subsequent inadequate (too short) hyperthermic phase (i.e., < 12 days) (2), by the method described in an earlier study (Table 1).3
Sexual behavior was scored as described previously. 4,5 Briefly, if the subject had sex at least once in each 7-day nonmenstruating week, her behavior was scored “weekly.” If the subject did not have heterosexual behavior each and every week but did record behavior during the course of the study, she was scored ”sporadic.” If she did not record any sexual behavior, she was scored “never.” Because we have previously shown4, 5 that intercourse and/or genital stimulation by a man qualifies as sufficient sexual behavior, whereas masturbation does not, we considered either of the two heterosexual behaviors adequate for counting as sexual behavior.
VALIDATION OF ACCURACY
At the conclusion of the study, subjects were administered a questionnaire to complete, which had a variety of questions designed to detect reliability (back-filling and omissions). Subjects were asked, for example, “How often during this study did you back-fill on BBT charts?” A 7-point Likert scale, from never to more than ten times, was provided for the subject. All subjects who back-filled any data were to be eliminated from the study, which would yield the 27 who completed the project. The procedure caused us to eliminate no subjects from the study.
STATISTICS
Data were analyzed with the use of four standard statistical procedures.
The t-test for comparison of differences between means was used to ask whether weekly active women had higher levels of sex steroid hormones than less sexually active women.
Correlation coefficients were used to ask whether increasing quantities of sexual behavior were associated with increasing quantities of hormones.
The Mann-Whitney U test, a nonparametric test that avoids the t-test’s assumption of a normal distribution, 16 was used to ask whether two independent groups were drawn from the same population and whether the distribution of sex steroid hormone scores among the weekly active women tended to be higher than among the less sexually active women.
The test of proportions17 was used to ask whether the proportion of women with aberrant menstrual cycles who had low estrogen was significantly greater than the proportion of women with normal menstrual cycles who had low estrogen levels.
RESULTS
Table 1 arrays the various data of the 27 subjects. Each hormone value, for each subject, represents the mean of her separate values. Table 2 provides the mean data (derived from individual data in Table 1) for the three steroids as well as age among the weekly, versus less than weekly, group. The T data of subject 5 is excluded, however, for reasons referred to earlier in Materials and Methods.
Table 2. Steroids and Age by Sexual Frequencya
Sexual Frequency |
Steroids |
Age |
||
T ng/ml |
P ng/ml |
E2 pg/ml |
||
| Weekly (n=8) | 0.86 + 0.07 | 5.73 + 0.78 | 118.75 + 12.67 | 25.63 + 0.60 |
Less than weekly |
0.83 + 0.04 | 6.22 + 0.27 | 65.84 + 2.56 | 21.74 + 0.25 |
| t = 4.02 P <0.01 |
t = 5.98 P <0.001 |
|||
a Values are + standard error.
As shown in Table 2, the T and P levels were not different in the two groups, whereas the E2 was significantly higher among the weekly subjects. We note also that the age of the weekly subjects was higher by 3.9 years. All subjects in this study had been menstruating for at least 7 years. Figure 1 arrays the E2 level by category of behavior. The weekly active women showed high levels of estrogen relevant to both the celibate and sporadic groups.
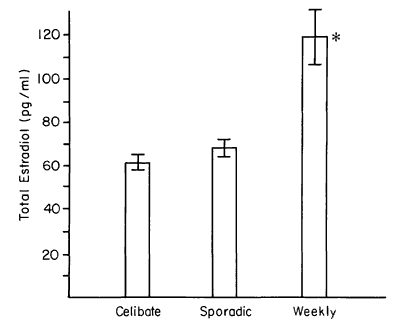
Figure 1 - E2 level was higher among weekly active women.
Figure 2 shows the E2 level of each subject as a function of her heterosexual total frequency for the duration of the experiment. Low estrogen levels, particularly values < 40 pg/ml, are common among sporadically active women. The possibility of a correlation between heterosexual total and each of the steroids was tested, but none was found. Consequently, total coital activity of a woman through the duration of the study was not related to her sex steroid hormone level. This lack of correlation may be visualized form Figure 2. Such was also the case in our study of perimenopausal women.7 Although the coital total does not appear to be related to the E2 level, examination of both Figures 2 and 3 suggests another subtle effect: that the distribution of E2 levels among the less than weekly women were lower than the distribution of E2 levels of the weekly active women. A Mann-Whitney U test was performed, which yielded a significant effect (U=42, P<0.025, 1 tail). This suggests that not only is the average level of one group different from the other but that the overall tendency is for lower levels of E2 to occur among women who have less than weekly sexual activity.
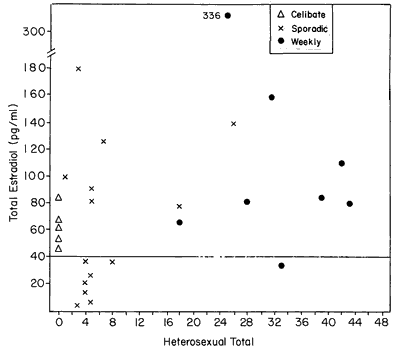
Figure 2 - E2 level as a function of sexual behavior. Although category of behavior (weekly, celibate, or sporadic) serves to distinguish women's behavior/E2 relationships, total quantity of sexual behavior does not correlate with E2 levels.
It may be noteworthy that none of the celibate women showed the extremely low levels of E2 that were characteristic of one half of the sporadically active women.
Figure 3 arrays the E2 level for each woman by her menstrual cycle length. The stipled region of the graph denotes the approximately 29.5+ 3-day cycle length.
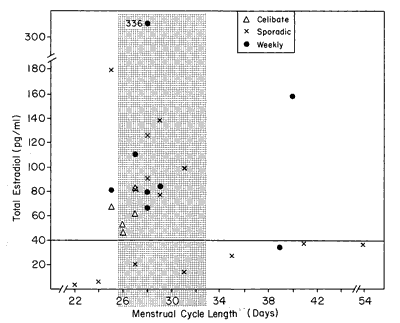
Figure 3 - Menstrual cycle length and E2 level. Women with low levels of E2 tend to show aberrant-length menstrual cycles.
A review of Figure 3 suggests that women who have aberrant menstrual cycle lengths, either less than 26 days or more than 33 days, have a tendency to have low E2 levels. This could be stated in a different way: that women who have high levels of E2 tend to have approximately 29-day menstrual cycle lengths. The test of proportions evaluated this question and did yield significant results (z= 2.10, P<0.02). Thus, the data in Figure 3 do indicate that aberrant-length menstrual cycles and low levels of E2 tend to occur together.
DISCUSSION
The present study suggests that women who have regular weekly sex with men have higher levels of estrogen than women who do not. Furthermore, it also suggests that the total amount of coital events is not relevant to the steroid level, whereas the consistency of the behavior is relevant. Although this result was implied by our earlier work on perimenopausal women, this is the first study in which hormone levels were concomitantly evaluated with the behavior and cycle length in women of reproductive age.
The finding that the weekly active group was slightly older than the less-active women is noteworthy. Particularly interesting is the fact that the P and T levels were not significantly different between the groups, whereas the E2 and age measures were. Whether the increasing age stimulates the potential for a more-active sex life or the increasing age is associated with a maturation of the estrogen-producing processes cannot be resolved by this study. However, there is reason to believe that the maturation has been already completed by the time of gynecologic maturity, more than 7 years since menarche has passed9, which was the prerequisite for this sample. The failure of the P or T to be distinguished by age supports the idea that the hormonal variation in E2 may be a sexual behavior associate, whereas sexual behavior may not alter P and T levels.
It has long been suggested that women with higher levels of T have higher levels of libido; however, this possibility was not directly addressed by the protocol of this particular study. Although we measured sexual behavior, we had no sensible way to measure desire for sexual activity. We do know from these data that women who have higher levels of estrogen tend to be the women who have weekly sexual activity and conversely, women who have weekly sexual activity tend to have higher levels of luteal phase estrogen. Since this association may be circular, we suggest that either hormones or behavior can affect each other.
Reproductive behavioral endocrinology has recently emerged into a field of inquiry directed toward the mammalian species. Within the past 25 years, numerous studies have been published that focus on a wide variety of infrahuman mammals.18 Although this is the first report of a relationship between reproductive behavior and reproductive hormones in cycling, presumably fertile women, the findings are not appreciably different from those that have been documented in other mammals.
References
1. Cutler WB, Garcia C-R, Kreiger A: Infertility and age at first coitus: a possible association. J Biosoc Sci 11:425. 1979
2. McFalls JA Jr: Impact of VD on the fertility of the U.S. black population. Soc Biol 20:2, 1973
3. Cutler WB, Garcia C-R, Krieger AM: Luteal phase defects: a possible relationship between short hyperthermic phase and sporadic sexual behavior in women. Horm Behav 13:214, 1979
4.Cutler WB, Garcia C-R, Krieger A: Sexual behavior frequency and menstrual cycle length in mature premenopausal women. Psychoneuroendocrinology 4:297, 1979.
5. Cutler WB, Preti G, Huggins GR, Erickson B, Garcia C-R: Sexual behavior frequency and biphasic ovulatory type menstrual cycles. Physiol Behav 34:805, 1985
6. Cutler WB, McCoy N. Davidson JM: Sexual behavior, steroids and hot flashes are associated during the perimenopause. Neuroend L 5:185, 1983
7. McCoy N, Cutler W, Davidson JM: Relationships among sexual behavior, hot flashes and hormone levels in perimenopausal women. Arch Sex Behav 14:385, 1985
8 Cutler WB, Garcia C-R., Krieger AM: Sporadic sexual behavior and menstrual cycle length in women. Horm Behav 14:163. 1980
9. Cutler WB, Garcia C-R: The Medical Management of Menopause and Premenopause: Their Endocrinologic Basis. Philadelphia, J.B. Lippincott, 1984
10. Vollman RF: The length of the premenstrual phase by age of women. In Proceedings of Fifth World Congress on Fertility and Sterility, Stockholm, Amsterdam, Excerpta Medica Int., No 133, 1968, p 1171
11. Vollman RF: Conception rates by days of the menstrual cycles, BBT, and outcome of pregnancy. Sixth World Congress of Gynecology and Obstetrics of the International Federation of Gynecology and Obstetric New York Abstracts. Baltimore, Williams & Wilkins, No. 112, 1970.
12. Vollman RF: The Menstrual Cycle. In Major Problems in Obstetrics and Gynecology. Philadelphia W.B. Saunders, 1977
13. Treloar AE, Boynton RE, Behn DG, Brown BW: Variations of the human menstrual cycle through reproductive life. Int J Fertil 12:77, 1967
14. Cutler WB, Preti G, Garcia C-R: Unpublished data
15. Katz Y, Dashow L, Epple A: Circulating steroid hormones of anadromous sea lampreys under various experimental conditions. Gen Comp Endocrinol 48:261, 1982
16. Siegel, S: Nonparametric Statistics for the Behavior Sciences. New York, McGraw-Hill, 1965
17. Dixon WJ, Massey SJ Jr: Introduction to Statistical Analysis, Third Edition. New York, McGraw-Hill, 1969. pp 249-250
18. Krieger DT, Hughes JC (Eds): Neuroendocrinology. Sunderland, Massachusetts, Sinauer Associates, Inc., 1980