Human Axillary Secretions Influence Women's Menstrual Cycles: The Role of Donor Extract of Females

George Preti,*++ Winnifred Berg Cutler,*+ Celso Ramon Garcia, ++ George R. Huggins,++ and Henry J. Lawley*
* The Monell Chemical Senses Center, 3500 Market Street, Philadelphia, PA 19104,
*+Athena Institute for Women's Wellness Research, 30 Coopertown Rd, Haverford PA 19104,
++Department of Obstetrics and Gynecology, School of Medicine, Hospital of the University of Pennsylvania, Philadelphia, PA 19104
Hormones and Behavior 20, 474-482 (1986)
Copyright. c 1986 by Academic Press, Inc.
Menstrual synchrony in human females has previously been demonstrated among women attending a predominantly female university as well as among women attending coeducational universities. In each of these studies, women who spent the most time together were most likely to shown the menstrual synchrony. In this experiment, the possibility that substances in axillary secretions might mediate this effect was tested using a prospective, double-blind research design and a combined axillary extract from a group of female donors. Female subjects who reported themselves to have normal (29.5+ 3 day) cycles were exposed to the axillary extracts or blank/ethanol for 10 to 13 weeks. Recipients of the axillary extracts showed a significant reduction in ”days' difference in menses onset” relative to the donor cycle, no change was evident for recipients of blank/ethanol. These results demonstrate that constituents from the axillary region of donor females can shift the time of menstrual onset of another group to conform with the donors' cycle and that this effect can occur even in the absence of social contact.
A number of exogenous influences have been shown to influence the length and timing of reproductive cycles in mammals (McClintock, 1983; Vandenbergh, 1983; Cutler and Garcia, 1980). Germane to the research reported here is the influence of females or their odors on the estrous cycle of other female conspecifics as this has been documented in recent studies employing nonhuman mammals (Izard and Vandenberg, 1982; McClintock, 1978). For example, the estrous cycle of rats can be manipulated through the use of odors generated from females during specific phases of the estrous cycle (McClintock, 1983, 1984). In addition, pre-ovulatory cervical mucus mixed with water and sprayed into the noses of a group of female Holstein cows advanced the time of estrous (Izard and Vandenbergh, 1982). Particularly intriguing has been the demonstration of menstrual synchrony in women; first, women attending a predominately female university (McClintock, 1971), and subsequently among women attending coeducational universities (Graham and McGrew, 1980; Quadagno, Shubeita, Deck, and Francoeur, 1981). In each of these studies, women who spent the most time together were most likely to show the menstrual synchrony.
The possibility that odors emanating from the axillary region might mediate these effects has been recently examined (Russell et al., 1980). Axillary secretions from a single female donor rubbed onto the upper lip of subjects caused the recipients to show a tendency toward menstrual synchrony with the donor cycle. In the pretreatment month recipients were, on average 9.3 days apart in menses onset form the donor's menstrual onset; after 4 months of treatment this difference was reduced to 3.4 days. However, this preliminary report has been criticized for lacking a double-blind research design (Doty, 1981); the technician who applied the odors was the same person who supplied the secretion.
The study reported here employed a double-blind research design and a combined axillary extract from one group of female donors. When this extract was applied to a separate group of the females, the recipients' cycles approached menstrual synchrony with the donor females.
METHODS
Donor secretions: Female extracts. Axillary secretions were collected from four female volunteer donors (ages 25, 26, 29, and 35) recruited from among co-workers and members of the community. All had the following characteristics: they were engaged in a heterosexual relationship; they had large numbers of lipophilic diphtheroids in their axillary region, and for the duration of the experiment they did not shave; they did not use deodorant, deodorant soaps, or perfumes in the axillary region; and they washed each morning with only Ivory soap. The odor which develops in the axillae has been shown to be a function of the resident microorganism present there (Labows, McGinley, and Kligman, 1982; Leyden, McGinley, Hoelzle, Labows, and Kligman, 1981). Correlations of odor quality and bacterial populations have been found in recent studies; these results show that when a faint or acid odor was present micrococcaceae were present in 100% of subjects. A more pungent odor similar to that of C19-16 androgen steroids is produced by the lipophilic diphtheroids, a different species of bacteria. These more pungent substances were found in 85% of males and 66% of females examined (Leyden et al., 1981). Our odor donors were picked for their ability to produce the complete spectrum of axillary odorants having both the lipophilic diphtheroids and the micrococcaceae as their resident microorganisms. The dominant constituents of both of these are currently thought to be both volatile acids and steroids.
Secretions were collected on 4x4 in. cotton pads which had been previously extracted, autoclaved, dried, and wrapped in solvent extracted aluminum foil (Preti and Huggins, 1975). Each donor wore a pad in each axillae three times a week during a convenient 6-to 9-hr period. After removal, pads were immediately frozen in acid/solvent cleaned glass jars and stored at -600C until extraction, approximately 1 year later. Each donor collected secretions for three complete menstrual cycles. However, the axillary pads included in the preparation of the stimulus came from only five of the donated cycles and represented all four donors which met the following more rigorous criteria: each was 29+2 days in length; the basal body temperature charts were clearly biphasic and presumably ovulatory with basal body thermal rises which lasted 12 or more days (Cutler, Garcia, and Kreiger, 1979; Cutler, Preti, Erikson, Huggins, and Garcia 1985; Treloar, Boynton, Behn, and Brown, 1967; Vollman, 1977); and menstruation occurred within 7 days of a full moon (Cutler, 1980; Friedman, 1981; Cutler, Schleidt, Friedman, Garcia, Preti, and Stine, 1987).
Because of the similarity in cycle lengths employed we made the assumption that each of the 3-day sequences from each donor (i.e., cycle Days 1, 2, and 3) would be endocrinologically similar and consequently combined the pads. To prepare the stimuli, pads were grouped in 3-day segments: e.g., all pads from cycle Days 1-3 were combined, and called “combined donor Day 2” (the first day of menstruation = Day 1 of a menstrual cycle); all pads in Days 4-6 were combined and called “combined donor Day 5”; all pads in Days 7-9 were combined to form “combined donor Day 8”, and so on. In this fashion, 10 separate extracts were prepared, each containing odors from different portions of the menstrual cycle to form the “donor cycle' of combined donor days 2, 5, 8, 11, 14, 17, 20, 23, 26, and 29. All pads from each 3-day group were placed in a glass column and allowed to soak in doubly distilled EtOH for 1 hr at room temperature. Fifteen milliliters of ethanol were used for each pad in the column. After 1 hr the ethanol extracted materials were allowed to run out the bottom of the column through a Teflon stopcock as the pads were squeezed with a Teflon disc. Approximately two-thirds of fall ethanol put on the pads was recovered. The ethanol extracted stimuli were subsequently frozen at -600C until needed.Subject Selection
Female subjects who were to receive the axillary extracts were recruited from among the members of the university community. The 19 subjects who completed the study ranged in age from 19 to 32. All met the following criteria: “gynecological maturity” (as defined by Treloar et al. (1967) as menstruating for at least 7 years), nulliparous, not currently (nor within the last 3 months) using oral contraceptives nor an I.U.D., and a willingness to make a daily entry of basal body temperature (BBT), sexual behavior, and menstrual occurrence. Each potential subject who met the criteria and indicated her interest in participating was asked whether her menstrual cycle was normal (29.5 + 3) or aberrant (<26 or >33 days) in length (Cutler et al., 1979, 1985; Vollman, 1977). Those who indicated a normal-type cycle length were included in this study; the women with aberrant cycle lengths were diverted to the “male axillary extract” experiment (Cutler, Preti, Huggins, Garcia, and Lawley, 1986). All subjects also agreed to provide a blood samples for steroid analysis during 3 days of one week in the luteal phase of the last menstrual cycle studied.Seasonal Constraints. This experimental protocol was designed to accommodate to the potential for a seasonal fluctuation in sexual cycles. Two relevant seasonal influences are the lunar and the annual geophysical cycles (for a brief review see Cutler, 1980). It has been shown that lunar and menstrual phase locking occurs (Cutler, 1980; Friedman, 1981). In the autumn, a maximum number of menses occurs concomitantly with the full moon (Cutler, 1980; Cutler et al., in preparation). For this reason we designed the experimental protocols in an attempt to minimize the potential influences of either of these geophysical effects on the experiment by (1) collecting extracts in the autumn of one year, storing them at -600C, and applying them (after thawing) in the autumn of the following year, and (2) selecting those autumn donors whose menses onset occurred within 1 week of the full moon.
Experimental protocol. Each subject was provided with BBT charts, BBT thermometers and a calendar card to record sexual behavior. Instructions for their use were given by a female technician who was blind to the purpose of the study. Subjects were unaware of the true nature of the stimuli; they were told that they were receiving “natural fragrance” extracted into ethanol. The informed consent form had two paragraphs pertaining to this 2. Thus, the study was double-blind. Subjects were randomly assigned to group C, female axillary extract/ethanol or group D, blank/ethanol. The average age of the C group (n=10) receiving female extract was 25.5 years while the average age of the D group (n=9) receiving blank/ethanol was 21.5 years.
Because of the individual variation in menstrual patterns, a woman could enter the study at any point within her cycle. Consequently if a woman entered the study on Day 10 of her cycle, she was scored as 9 days out of phase with the first application of the donor extract. If she entered the study on day 20 of a 29 day cycle she was also scored as 9 days out of phase with the first application of the donor extract. Upon entry, to the study, women received female extract from “combined donor Days 2” (i.e., pooled sample from cycle days 1, 2, and 3). Sequential preparations of the remaining extracts were applied during subjects' subsequent visits to the laboratory (e.g., on her next visit “combined donor Days 5” was applied; on her next visit “combined donor days 8” extract and so on until her 11th visit to the laboratory, when the sequential process began again and she received combined donor Day 2 once again. Consequently, the 11th application (i.e., combined donor Day 2 extract for subjects or ethanol for controls) was given to women in groups C and D, respectively, every 22-25 days.
Subjects came into the laboratory three times each week for 10 to 13 weeks. Subjects in group C received an application of the timed female extract, subjects in group D received extract (or blank/ethanol). Substance was applied in the following manner.
Each morning the stimuli to be used that day were removed from the freezer and allowed to warm to room temperature for 30 min. Individual 5-ml samples of stimuli and blank (ethanol) were then removed via pipet and placed in separate vials labeled C and D, respectively, and transported to the Ivy Research Laboratory where the stimuli were applied. The technician was instructed which subject was to receive which vial contents. The Ivy Laboratory is in a building some distance from the Monell Center; consequently, neither the technician nor the subjects came to the Monell Center. The technician pipetted 0.5 ml of the stimuli onto a clean 4x4 in. cotton pad and rubbed the contents of the pad onto the upper lip of the subject and instructed her not to wash the area for at least 6 hr. She then recorded the blood pressure and pulse of the subject in an attempt to dilute the focus of the study. Neither of the experimenters handling the extracts on a routine basis (G.P.; H.J.L.) could distinguish a difference in odor between the blank and female extracts. Following completion of the experiment, the technician said she could not tell the difference between C and D samples, nor had any of the subjects commented on an odor from either sample.
RESULTS
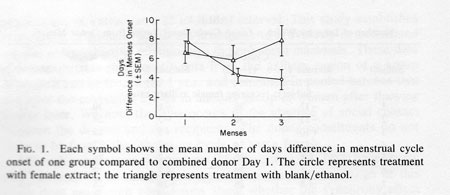
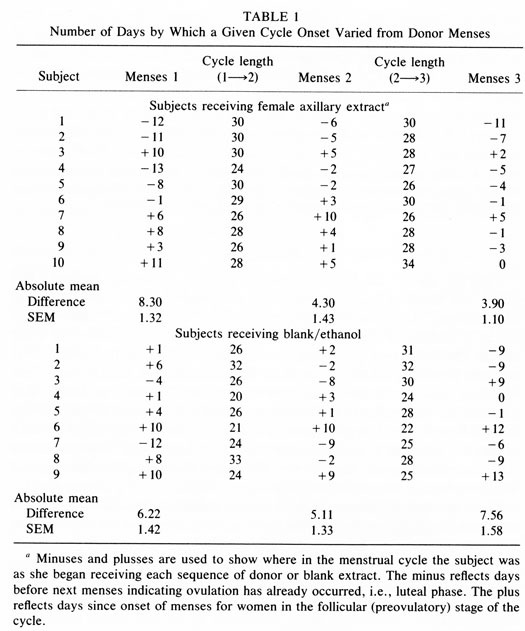
Figure 1 shows the average number of days difference between the menstrual bleeding onset of members of group C and of group D and the “menses'” (combined donor Day 1) of the “donor cycle” during each of the three months of the study. Table 1 lists each woman's data for each of the three menses occurring during the participation in the study. For subjects receiving extract application the differences in days of menstrual bleeding onset from the combined donor Day 2 extract is listed. For controls the difference between menses onset and the start of each series of 10 blank/ethanol applications is listed. In the first cycle, the mean difference in group C menses onset to the “donor cycle” menses onset (i.e. combined donor Day 2) was 8.3 days. For group D this was 6.2 days, both near the expected difference of 7.38.
The expected difference of 7.38 days is calculated as follows: since a criterion for inclusion in the study was a cycle length of 29.5 + 3 days, any two menses onsets will average somewhere between 0 and 14.75 days apart (half of 29.5). As a consequence, the theoretical average difference is 7.38 days (half of 14.75). Group C at 8.3 days and Group D at 6.2 days reflect this tendency. After 2 more months of female extract application the number of days' difference in menses onset relative to the “donor cycle” was significantly reduced (by about 50%) for group C from 8.3 to 3.9 days while no change was evident for group D (Fig. 1). An analysis of variance with repeated measures showed a significant month x group interaction F (2, 34) = 5.09; P < 0.05. No other term was significant in the analysis. Post hoc tests were used to explore the source of interaction more fully. F tests were run separately for the experimental and control groups. Only the experimental group showed the significant shift in menses onset during the periods monitored [F(2, 18) = 7.53; P < 0.01]; the control group showed no such effect [ F(2, 18) = 1.82; P = 0.20]. Thus, only the experimental group showed a change in cycle timing reflective of synchrony. Post hoc t tests were also performed; they revealed that the control and experimental groups differed only in the third menses period (t17 = 2.04; P < 0.05).
Blood samples drawn during the last cycle of participation, showed no apparent difference in the levels of estradiol, testosterone, or progesterone between groups C and D when controlled for time of cycle.
DISCUSSION
This study represents the first systematically designed, prospectively conducted, double -blind research in humans to attempt to manipulate the menstrual cycle with female-derived secretions. In this experiment naturally occurring 29.5 + 3 day cycles could be modulated with repeated applications of extract at a 22 to 25 day interval. This study establishes phenomena in humans which are analogous to previously demonstrated olfactory/reproductive relationships in nonhuman mammals. These data also demonstrate that constituents from the axillary region of a group of women can be frozen for 1 year and combined in pooled batches that can alter the menstrual cycles in another group of women after thawing 1 year later. We note that this occurs in the absence of social contact between the donors and the recipients. The donor constituents do not have to be from either the same cycle or same female.
Although experiments with rodents (McClintock, 1983, 1984) have shown that airborne odors can produce estrous synchrony, the design of this study does not permit conclusions about whether the synchrony effect is mediated by olfaction or by absorption of the correct constituents. However, the subjects in this study worked and/or attended classes in a heterosexual environment. All were either engaged in or were “in between” a heterosexual relationship and presumably had a close circle of female friends which could influence cyclicity. Despite this, the cyclicity of subjects receiving combined female extract was rapidly altered well within the 3-4 months discussed by McClintock (McClintock, 1984) or the 4-5 months shown by Russell's subjects (Russell, Switz, and Thompson, 1980). It is of interest to note that an ovarian follicle requires approximately 85 days for maturation (Gougeon, 1982), which parallels the time needed to see a significant trend toward synchrony in this study.
The short time required to demonstrate synchrony with the donor cycle suggests that using a stimulus from a group of donors may provide a stronger stimulus than using the stimulation of a single donor (Russell et al., 1980). Thus, in the strength and frequency used here, application of a combined female axillary extract can alter the menstrual cycles of normal cycling women.
ACKNOWLEDGEMENTS
This study was supported in part by a grant from the National Science Foundation, BNS 82-03018. The authors thank Gary K. Beauchamp and Carol Christensen for their constructive comments concerning the mansucript, Mrs. Helen Liebich for her technical assistance, and Mrs. Janice Blescia for processing the manuscript. Dr. James Leyden, Dr. Ken McGinley, and Dr. Joseph Nicholson of the Duhring Laboratories are thanked for their screening of potential subjects for the correct axillary microflora and odor production.
REFERENCES:
Cutler, W.B.,(1980). Lunar and mesntural phase locking. Amer.J.Obstet.Gyencol. 137, 834-839.
Cutler, W.B., and Garcia, C.R. (1980). The psychoneuroendocrinology of the ovulatory cycle of women. Psychoneuroendocrinology.5(2), 89-111.
Cutler, W.B., and Garcia, C.R., and Kreiger, A.M. (1979). Sexual behavior frequency and menstrual cycle length in mature premenopausal women. Psychoneuroendocrinology. 4, 297-309.
Cutler, W.B., Preti, G., Erikson, B., Huggins, G., and Garcia, C.R. (1985). Sexual behavior frequency and biphasic ovulatory type menstrual cycles. Physiol. Behav. 34, 805-810.Cutler, W.B., Preti, G., Huggins, G.R., Garcia, C.R., and Lawley H.J. (1986). Human axillary secretions influence women's menstrual cycles: The role of donor extract of men. Hormon. and Behav.
Cutler, W.B., Schleidt, W.M., Friedman, E., Preti, G., Garcia, C.R. and Stine, R. (1987). Lunar influences on the reproductive cycle in women. Submitted for publication.
Doty, R.L., (1981). Olfactory communication in humans -- a review. Chem. Senses 6(4), 351-376.
Friedman, E. (1981). Menstrual and lunar cycles. Amer. J. Obste. Gynecol. 140, 350.
Gougeon, A., (1982). Rate of Follicular Growth in the Human Ovary. In R. Rolland, E., van Hall, S.G. Hillier, K.P. McNatty, and J. Schoemaker (Eds.), Follicular Maturation and Ovulation, pp. 155-163 Excertpa Medica, Princeton.
Graham, C.A., and McGrew, W.C., (1980). Menstural synchrony in female undergraduates living on a co-educational campus. Psychoneuroendocrinology.5, 245-252
Izard, M.K., and Vandenbergh, J.G. (1982). Priming pheromones from oestrous cows increase synchronization of estrous in dairy heifers after PGF-2 injection. J. Reprod. Fertil. 66, 189-196.
Labows, J.N, McGinley, K. J., and Kligman, A.M. (1982). Perspectives on axillary odor. J. Soc. Cosmet. Chem. 34, 193-202.
Leyden, J.J., McGinley, K.J. Hoelzle E., Labows, J.N., and Kligman, A.M.(1981). The microbiology of the human axillae and its relation to axillary odors. J. Invest. Dermatol. 77, 413-416.
McClintock, M.K., (1971). Menstrual syncrhony and suppression. Nature (London) 229, 244-245.
McClintock, M.K. (1978). Estrous synchrony and its mediation by airborn chemical communication (Rattus norvegicus). Horm. Behav. 10, 264-276.McClintock, M.K. (1983) Pheromonal regulation of the ovarian cycle: Enhancement suppression and syncrhony. In J.G. Vandenbergh, (ed)., Pheromones and Reproduction in Mammals, pp. 113-149. Academic Press, New York.
McClintock, M.K., (1984). Estrous synchrony: Modulation of ovarian cycle length by female pheromones. Physiol. Behav. 32, 701-705.
Preti, G., and Huggins, G.R. (1975). Cyclic changes in volatile acidic metabolites of human vaginal secretions and their relation to ovulation. J. Chem. Ecol. 1, 361-376.
Quadagno, D.M., Shubeita, H.E., Deck, J., and Francouer, D. (1981). Influence of male social contacts, exercise and all female living conditions on the menstrual cycle. Psychoneuroendocrinology. 6, 239-244.
Russell, M.J., Switz, G.M., and Thompson, K. (1980). Olfactory influences on the human menstrual cycle. Pharmacol. Biochem. Behav. 13, 737-738.
Treloar, A.E., Boynton, R.E., Behn, D.G., and Brown, B.W. (1967). Variation of the human menstrual cycle through reproductive life. Int. J. Fertil. 12, 77-126.
Vandenbergh, J.G. (Ed.) (1983). Pheormonal regulation of puberty. In Pheromones and Reproduction in Mammals, pp. 113-149. Academic Press, New York.
Vollman, R.F. (Ed.) (1977). The menstrual cycle. In Major Problems: Obstetrics and Gynecology, Vol. 7. Saunders, Philadelphia.