Human Axillary Secretions Influence Women's Menstrual Cycles: The Role of Donor Extract from Men

Winnifred Berg Cutler,*+ George Preti,*++ Abba Kreiger, &, George R. Huggins,++ Celso Ramon Garcia, ++ and Henry J. Lawley*
*+Athena Institute for Women's Wellness Research, 30 Coopertown Rd, Haverford PA 19104
* The Monell Chemical Senses Center, 3500 Market Street, Philadelphia, PA 19104.
++Department of Obstetrics and Gynecology, School of Medicine, Hospital of the University of Pennsylvania, Philadelphia, PA 19104
& Dept of statistics, The Wharton School, University of Pennsylvania, Philadelphia, PA.
Hormones and Behavior 20, 463-473 (1986)
Copyright. c 1986 by Academic Press, Inc.
Menstrual cycle lengths of 29.5 + 3 days (“normal cycles”) are more frequent in women who have weekly coital activity than in women who do not. In order to investigate potential mechanisms controlling the association between heterosexual activity and menstrual cycle length, and in light of the nonhuman literature suggesting that a chemical signal from males could be involved, menstrual cycle lengths of nulliparous women were evaluated following regular application of axillary extract from donor males. Compared to controls receiving only blank/ethanol applications, women receiving axillary extracts for 12.5 to 14.5 weeks showed the following changes: (1) reduced incidence in variability of cycle lengths; and (2) a reduced proportion of aberrant length cycles.
c. 1986 Academic Press, Inc.
Studies in several mammals have shown that the presence of a male or his odors influences the reproductive physiology of the female (Izard, 1983; McClintock, 1983; Richmond and Conoway, 1969). For example, in several rodent species, exposure to the odors of urine or cage bedding of males can promote estrus or stimulate ovulation (Marsden and Bronson, 1964; Whitten, Bronson, and Greenstein, 1968). In some infrahuman primates, males can influence cycle length. Female baboons denied mating show significantly longer cycles (Howard-Tipp and Bielert, 1978) and rhesus monkeys show a summer amenorrhea 2 to 4 months after a male decrease in sexual potency (Michael and Zumpe, 1976).
In humans, studies by several investigators (McClintock, 1971; Cutler, Garcia, and Kreiger, 1979; Cutler, Preti, Erickson, Huggins, and Garcia, 1985; Veith , Buck, Getzlaf, Van Dolfsen, and Slade, 1983) have shown that women who spend more time with men have a greater probability of having regular (29.5 + 3 day) menstrual cycles. Cycle lengths of 29.5 + 3 days have the highest likelihood of normal biphasic basal body temperature rhythms (Treloar, Boynton, Behn, and Brown, 1967; Vollman, 1977). A recent study demonstrated that sex with men, when it occurs in a consistent and regular pattern increases, or is associated with an increased incidence of, normal biphasic type basal body temperature rhythms (Cutler et al., 1985). In contrast, consistent masturbation (i.e, weekly or more) was not associated with an increased incidence of 29.5 + 3 days cycles. In combination, these studies show that women who have regular (at least weekly) sexual activity with males are more likely to show presumptively biphasic ovulatory type patterns of menstrual cyclicity than women whose sexual activity with males is either infrequent or nonexistent.
Regular sexual behavior and normal biphasic menstrual cycle patterns occur together (Cuter et al., 1985). That regular sexual behavior leads to regular cycle lengths can be inferred from published data by noting that (1) women with a pattern of weekly heterosexual behavior consistently showed a high incidence of “normal” (29.5 + 3 days) cycles, fertile-type basal body temperature rhythm, and higher postovulatory estrogen levels, but (2) the reverse situation - by the presence of a normal cycle length, a normal biphasic basal body temperature rhythm, or higher postovulatory estrogen levels did not necessarily suggest which pattern of sexual behavior would exist (Cutler, Davidson, and McCoy, 1983; Cutler, Garcia and Kreiger, 1979a; Cutler et al., 1985; Cutler, Garcia, Huggins, and Preti, 1986). Therefore, we have previously suggested that the pattern of weekly sex may have some actual influence on the underlying endocrine milieu of women (Cutler et al., 1985, 1986). Moreover, the physical presence of a man appears to be necessary, but the actual act of coitus may not be, provided there is genital stimulation; since masturbation is not, while genital stimulation (short of coitus) by a male is, effective as an associate of biphasic ovulatory type cycle). In order to investigate potential mechanisms controlling the association between heterosexual activity and menstrual cycle length, and in light of nonhuman literature suggesting that a chemical signal from males could be involved, we investigated whether an extract from the male axillary region would be a sufficient stimulus to induce these presumptively biphasic ovulatory type cycles. We now report evidence indicating that it can.
METHODS
Seasonal constraints. This experimental protocol was designed to accommodate the potential for a seasonal fluctuation in sexual cycles. Two relevant seasonal influences are the lunar and annual geophysical cycles. In women, it has recently been shown that lunar and menstrual phase locking occurs. A maximum in number of menses occurred within a week of the full moon in the autumn in 3 separate years (Cutler, 1980; Friedman, 1981; Cutler, Garcia, Preti, and Stine, in preparation). In men, an annual variation in testosterone rhythms was reported with peak levels in autumn (Baker, 1975; Doering et al., 1975). Therefore, in order to minimize the potential influences of either of these geophysical effects on the experiment, male extracts were collected in the autumn of one year, frozen, and applied (after thawing) in the autumn of the following year.
Donor secretions: Male extract. Axillary secretions were collected from three male volunteers (ages 41, 35, and 32). Each was engaged in a heterosexual relationship and had large numbers of lipohilic diptheroids in his axillary region (Labows, McKinley, and Kligman, 1982). The quality of the odor which develops in the axillae has been shown to be a function of the resident microorganisms present there. Correlations of odor quality and bacterial population show that micrococcaceae were present in 100% of subjects when a faint or acid odor was present (Leyden, McGinley, Heolzle, Labos, and Kligman, 1981). In comparison, the lipophilic diptheroids were associated with a more pungent odor. These were found in 85% of the males and 66% of the females examined. This pungent odor is similar to that of C19-1*16- androgen steroids such as androstenone. Consequently, our extract donors, who had lipophilic diphtheroids and micrococcaceae as their resident microorganisms were selected for their ability to produce the complete spectrum of axillary odorants, the dominant constituents of which are currently thought to be volatile acids and volatile steroids. Secretions were collected in the autumn of 1982 and applied in the autumn of 1983.
During the 3 months in which donors collected secretions they were required to (1) not use deodorant, deodorant soap, or perfumes in the axillary region and (2) wash once in the morning with Ivory soap. Secretions were collected on 4 x 4 in. cotton pads, which had been previously extracted, autoclaved, dried, and wrapped in solvent extracted foil (Preti and Huggins, 1975). Each donor wore a pad in each axillae three times a week during a 6- to 9-hr period which was most convenient for him. Each day after removal, the pads from any one donor were placed in an individual acid-cleaned glass jar and frozen at 60oC until extraction. Secretions were collected 3 times a week from each donor for a 12-week period.
Preparation of extracts were conducted after all pads had been collected and frozen. Batches of glass jars were grouped consecutively according to the date received. Six to eight jars containing pads from all three individuals were used to prepare each batch of pooled extract. A total of 14 separated batches were prepared in this manner. All pads from each were placed in a glass column and allowed to soak in doubly distilled ethanol (15ml/pad) for 1 hr. The ethanol was allowed to drain from the column through a Teflon stopcock and the pads were squeezed with a Teflon disk. Recovery approached 66% of the ethanol added to the pads. Presumably the remaining 34% remained within the pads. The ethanol extracted stimulus batches were then stored at - 60°C.
Subject selection. Assignment of subjects to the Female Extract Experiment (Preti, Cutler, Huggins, Garcia, and Lawley, 1986) or the Male Extract Experiment was made after asking each potential subject to estimate her previous cycling pattern. Women who believed themselves to cycle within the 26 to 32 day range were assigned to the Female Extract Experiment (Preti et al., 1986); women who believed themselves to have aberrant length menstrual cycles (<26 or >32 days) were assigned to the Male Extract Experiment reported here. Subsequently 15 women, varying in age from 19 to 22 and one aged 30 were enrolled in and completed the male extract study. All met the following criteria: “gynecological maturity” (as defined by Treloar eat al., 1967 , as menstruating for at least 7 years), nulliparous, unmarried, not currently (nor within the last 3 months ) using oral contraceptives nor an I.U.D. and a willingness to make daily entry of basal body temperature (BBT) and sexual behavior. In addition, all subjects agreed to provide blood samples for steroid analysis during 3 days of 1 week in the luteal phase of the last menstrual cycle studied. A complete history and physical exam was performed on each subject by one of us (G.R.H.). This screening process for possible pathologies which might influence the length of the menstrual cycle failed to find any.
The experimental protocol. Each subject was provided with BBT charts, BBT thermometers, and a calendar card to record sexual behavior, cycle length, and menstruation. Instructions for their use were given by a technician who was blind to the purpose of the study. Subjects were unaware of the true nature of the stimuli, and were only told that they were receiving a "natural fragrance” extracted into ethanol. Thus the study was double-blind. Subjects were randomly assigned to group A (axillary extract/ethanol) or group B (blank/ethanol). The average age of the A group was 20 while the average age of the B group was 22. Application of the stimuli began in September, 1983, but because of individual variation in menstrual patterns, any particular woman could enter the study at any point within her cycle.
The technician was housed in an office in the Ivy Research Laboratories which is several buildings removed from the Monell Center. A blind test at the end of the experiment revealed that personnel at the Monell Chemical Senses Center as well as the technician working at Ivy Labs were unable to distinguish fresh samples “A” from “B” when tested. The technician also noted that none of the subjects in the study had commented on either A or B having an odor. Subjects came to this laboratory three times each week for 12.5 to 14.5 weeks for application of the stimuli (male extract or placebo). Each morning the previously frozen stimuli were removed and allowed to warm to room temperature for 30 min. Individual 5-ml samples were removed via pipet and placed in separate vials (labeled A and B) for transport to the laboratory where the stimuli were applied. One-half milliliter of the stimuli (axillary extract or blank/ethanol) was pipetted onto a clean 4 x 4 in. cotton pad. The technician rubbed the contents of the pad on the upper lip of the subject and instructed her not to wash the area for at least 6 hours. She then recorded the blood pressure and pulse of the subjects in an attempt to dilute the focus of the study.
Treatment began in the third week of September as subjects were enrolled into the study. The first menses thereafter and the one which followed this formed the boundaries of “first cycle” of the experimental period. A typical example then, is represented as follows: …9/22 (enter study, treatment begins)…10/4 (first menses onset)…11/8 (second menses onset)…12/4 (third menses onset)…12/12 (treatment ends). In this case, two cycles of 35 and 26 days would form the first and the last cycle length of this subject.
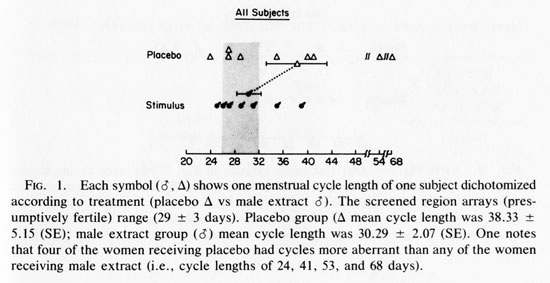
RESULTS
Figure 1 shows the last cycle length of all subjects receiving stimulus or placebo. The last cycle of each subject was selected for data presentation and analysis since this would allow the maximum time to test for an effect during this 14-week experiment. One-tailed tests define all probability levels because a prediction - that male extracts will reduce the incidence of aberrant length menstrual cycles -is fundamental to this experiment. The mean cycle length of the two groups were not significantly different (T= 1.45, p <0.10).
An initial glance at Fig. 1 seems to suggest that stimulus recipients have less variance in their cycle length about their own means and a greater incidence of cycle lengths that occur within the screened area, i.e., 29.5 + 3 days. In order to test these observations, it is useful to distinguish (1) decreased variability in cycle length from (2) increases incidence of normal (29.5 + 3day) length menstrual cycles.
We ran the Mann-Whitney U test (Dixon and Massey, 1969) on the absolute deviations from the mean of each group. This was done to test whether the placebo population had greater variability in cycle length. The results were significant U= 15; (P<0.05). When the Mann-Whitney U test is run on the absolute deviations from the grand mean, including women with weekly sexual activity, the results fail to achieve significance (U= 21, P< 0.05).
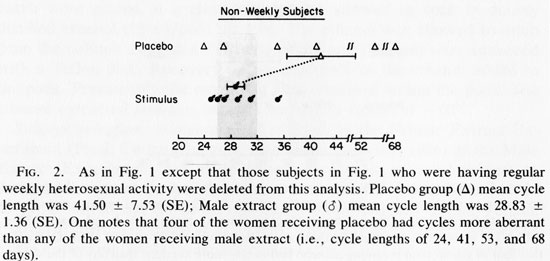
Because weekly coital behavior and 29 day cycles were associated (Cutler et al., 1979a, b, 1985; McCoy et al., 1985) we removed the data of those women who were having weekly coital activity with men from the data set and reanalyzed the data (see Fig. 2). The mean cycle lengths again were not significantly different (t=1.645, 0.05 <P <0.10). The Mann-Whitney U test was again applied to the absolute deviations from the mean of each group. This showed significantly less variability in cycle length for stimulus recipient (U= 5, P<0.02). When the Mann-Whitney U test was rerun with the more conservative method using the absolute deviations from the grand mean, the results again achieved significantly less variance among extract recipients (U= 6, P < 0.03). The analysis of these data which evaluate data variance relationships suggests that variance in cycle length is reduced by male extract treatment, when no regular heterosexual behavior is occurring. Analysis of the biologically more relevant question: Does male extract reduce the proportion of aberrant length cycles?, suggests that it does this as well.
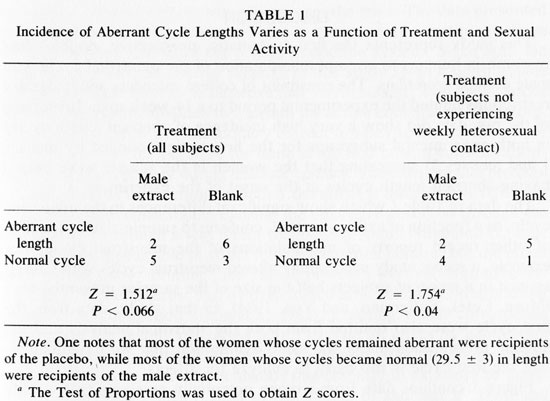
Table 1 shows the incidence of aberrant length cycles (<26 or > 32 days) to vary as a function of treatment in all women (left) and when controlled for sexual activity (right). In studying either all the subjects, or only those subjects who were not having weekly sexual activity, exposure to male axillary secretions yielded a greater proportion of "normal" (29.5 + 3 day) cycle lengths than exposure to the blank (Test of Proportions, Z = 1.512, P < 0.066 all subjects and Z = 1.754, P < 0.04 subjects who are not sexually active weekly).2 There were no apparent differences in testosterone, progesterone, or estradiol levels among women who received different treatments.
2. In order to apply appropriate statistical tests to these data, several considerations are relevant. While at first glance, one might consider Fisher's Exact Probability test (Siegel, 1956) the appropriate one, this is not so for several reasons. Fisher's Exact Probability test is a finite population test which means in this context, that two prerequisites hold: (A) the number of individuals receiving the stimulus and placebo is prefixed by the research design (true of this experiment) and (B) the number of individuals with aberrant and normal length cycles are prefixed by the design (a condition which is not true of this experiment). In contrast, the Test of Proportions (Dixon and Massey, 1969) only assumes condition A, that the experimental design predetermines the number of women assigned to each stimulus or placebo group. Condition B, the number of aberrant cycles might very well vary in a replication of this experiment. For this reason, Fisher's Exact Test is inappropriate and the Test of Proportions (Dixon and Massey, 1969) was applied.
DISCUSSION
This study represents the first systematic, prospective, double-blind research in humans to attempt manipulation of the menstrual cycle with male derived secretions. The constraint of college calendars and budgetary restriction limited the experimental period to a 14-week span. Inspection of the raw data did show a very high incidence of aberrant length cycles in both experimental subgroups for the first cycle (bounded by menses 1 and menses 2) suggesting that the women in the sample were indeed having aberrant length cycles at the onset of the experiment.
The data in Table 1 which show significant differences in the menstrual cycle, as a function of axillary extract, conform to sample-size constraints of other recent reports of manipulations of the menstrual cycle. For example, a recent study successfully altered menstrual cycles with GnRH-agonist in a group of subjects half the size of the samples presented here (Muse, Cetel, Futterman, and Yen, 1984). In that study, data from the first cycle were also omitted from both the statistical analysis and the graphic presentation under the same assumption that we have made - that the first cycle is too early to analyze for effects.
Figure 1 contains data from women potentially receiving two weekly influences: male extract and/or heterosexual behavior in weekly frequencies. Heterosexual behavior (see studies cited in Introduction), or male extract for those women not experiencing weekly sex (see Fig 2 and Table 1, right column) appear adequate to limit the incidence of aberrant menstrual cycle lengths. Therefore, the data in Figure 1 may contain the influence of weekly heterosexual contact which masks the influence of male extract. Figure 2 therefore, shows the potential for male extract effects without the confounding variable of weekly heterosexual exposure. One should note that while “mean cycle length” is an insensitive indicator of the effect under consideration, the variance about the mean is quite sensitive to the question of fertility potential (Cutler et al., 1979a, 1976; Cutler and Garcia, 1980; McCoy et al., 1985). A group of women with aberrant cycle lengths can share a mean cycle length in the normal (29.5 +3 day) range while every individual comprising the data base shows an aberrant length. If one were to focus on differences between the mean cycle length of the experimental and control groups, one might overlook this essential point. The results of this initial experiment have shown that although male extracts do not change the mean cycle lengths, they do reduce the proportion of aberrant length cycles within 14 weeks of treatment.
The possible role that odors plays in human reproductive biology has received considerable attention particularly with respect to the extent of which menstrual synchrony may occur in females who live together. Discussions of male-female effects have often centered on the possibility that odors act to attract, or sexually arouse, the male. The data presented here suggest that prolonged exposure to one or more constituents from the male axillae may alter the female endocrine system. Since both the experimental and the control subjects in this study spent a considerable portion of their day in a heterosexual environment, the usual social distance appears inadequate to transmit the active constituent. Therefore, the causative factor in male axillary extract is likely to have a low vapor pressure at body temperature (or none at all) and critical concentrations may be transferred only during intimate contact. The experimental protocol did not allow us to determine whether the effect is mediated by olfactory stimuli or if the constituents causing the effect are absorbed through the skin and thus through a less direct (endocrine) route. Nonetheless, male extract does appear able to affect female menstrual pattern; but the effect may require some "priming time” since 14 weeks were required to reveal the effect. It is of interest that ovarian follicular development needs about 85 days for completion (Gougeon, 1982), a timing which corresponds nicely with the results discussed here. We find an effect by completion of the third cycle (Gougeon, 1982). In addition, this time requirement supports earlier tentative conclusions that stability in exposure to males affects menstrual cycles (Cuter et al., 1979a, b, ) and that a "feast and famine" approach to coital exposure may disrupt the menstrual cycle (Cutler et al., 1980, 1986).
This is the first double-blind perspective investigation of the potential for male extract to manipulate women's menstrual cycles. Because human experiments are extremely costly in both time and money it is appropriate that these initial results be recorded in the hope that these studies will stimulate further research by other groups. Future efforts to replicate these findings should employ a control cycle with placebo before the experimental procedure begins in order to confirm that all participants have aberrant length cycles before they begin the treatment protocols.
ACKNOWLEDGEMENTS
This study was supported in part by a grant from the National Science Foundation, BNS 82-02018. We thank Dr. Gary K. Beauchamp and Dr. Charles Wysocki for their constructive comments concerning the manuscript, Mrs. Helen Leibinch for her technical assistance, and Mrs. Janice Blescia for processing the manuscript. Dr. James Leyden, Ken McGinly, and Joseph Nicholson of the Durhing Laboratories are thanked for their screening of potential subjects for the correct axially microflora and odor production.
REFERENCES
Abrahamson, G.E., Maroulis, G.B.,, and Marshall J.K. (1974). Evaluation of ovulation and corpus luteum function using measurements of plasma progesterone. Obstet. Gynecol. 44, 522-525.
Baker, H.W.G., et al. (1975). Changes in the pituitary testicular system with age. Clin. Endocrinol., 5, 349-372
Cutler, W.B., (1980). Lunar and menstrual phase locking. Amer. J. Obstet. Gynecol. 137, 834-839.
Cutler, W.B., Davidson, J. M., and McCoy, N. (1983) Relationships between estrogen level, hot flashes, and sexual behavior in menopausal women. Neuroendocrinol. Lett. 5 (3), 185.
Cutler, W.B., Garcia, C.R. (1980). The psychoneuroendocrinology of the ovulatory cycle of women. Psychoneuroendocrinology (5)2, 89-111.
Cutler, W.B., Garcia, C.R., and Kreiger, A.M. (1979a). Sexual behavior frequency and menstrual cycle length in mature premenopausal women. Psychoneuroendocrinology, 4, 297- 309.
Cutler, W.B., Garcia, C.R., and Kreiger, A.M, (1979b) Luteal phase defects: A possible relationship between short hyperthermic phase and sporadic sexual behavior in women. Horm. Behav., 13, 214-218
Cutler, W.B., Garcia, C.R., and Kreiger, A.M., (1980) Sporadic sexual behavior and menstrual cycle length in women. Horm. Behav. 14, 163-172.
Cutler, W.B., Garcia, C.R., Huggins, G.R., and Preti, G. (1986) Sexual behavior and steroid levels among gynecologically mature premenopausal women. Fertil. Steril. 45, 496-502.
Cutler, W.B.,, Preti, G., Erickson, B. Huggins, G.R., and Garcia, C.R., (1985) Sexual behavior frequency and ovulatory biphasic menstrual cycle patterns. Physiol. Behav. 34, 805-810
Dixon, W.J. and Massey, S.J., Jr. (1969). Introduction to Statistical Analysis, 3rd ed., pp. 249-250. McGraw Hill, New York.
Doering, C. et al. (1975). A cycle of plasma testosterone in the human male. J. Clin. Endocrinol. Metab. 40, 497.
Friedmann,E. (1981). Menstrual and lunar cycles. Amer. J. Obstet. Gynecol. 140, 350.
Gougeon, A. (1982). Rate of Follicular Growth in the Human Ovary. In R. Rolland, E.V. van Hall, S.G. Hillier, K.P. McNatty, and J. Schoemaker (Eds.), Follicular Maturation and Ovulation, pp. 155-163. Excerpta Medica, Princeton.
Howard-Tipp, M.E., and Bielert, C. (1978) Social contact influences on the menstrual cycle of the female chacma baboon (Papio ursinus). J.S. Afr. Assoc. 49, 191-192.
Izard, M.K., 1983. Pheromones and reproduction in Domestic Animals. In J.G. Vandenbergh (Ed.), Pheromones and Reproduction in Mammals, pp. 253-281. Academic Press, New York.
Labows, J.N., McKinley, K.J., and Kligman, A.M. (1982). Perspectives on axillary odor. J. Soc. Cosmet. Chem. 34, 193-202.
Leyden, J.J. McGinley, K.J., Hoelzle, E., Labows, J.N., and Kligman, A.M. (1981). The microbiology of the human axillae and its relation to axillary odors. J. Invest. Dermatol. 77, 413-416.
Marsden, H.M., and Bronson F.H. (1964). Estrous synchrony in mice: Alteration by exposure to male urine. Science 144, 1469.
McClintock, M.K., (1971).. Menstrual synchrony and suppression. Nature (London) 229, 244-245.
McClintock, M.K., (1983). Pheromonal Regulation of the Ovarian Cycle: Enhancement, Suppression and Synchrony. In J.G. Vandenbergh (Ed.), Pheromones and Reproduction in Mammals, pp. 113-149. Academic Press, New York.
McCoy, N., Cutler, W., and Dawson, J.M. (1985). Relationships among sexual behavior, hot flashes and hormone levels in perimenopausal women. Arch. Sex. Behav. 14, 385-394
Michael, R.P., and Zumpe, D. (1976). Environmental and endocrine factors influencing annual changes in sexual potency in primates. Psychoneuroendocrinology.1, 303-313
Muse, K.N., Cetel, N.S. Futterman, L.A., and Yen, S.S.C. (1984) The premenstrual syndrome: Effects of “medical ovariectomy.” N. Engl J. Med. 311, 1345-1349.
Preti, G., Cutler, W.B. Huggins, G.R. Garcia C.R. and Lawley, H.J. (1986). Human axillary secretions influence women's menstrual cycles: The role of donor extract from women. Horm.Behav. 20.
Preti, G., and Huggins, G.R. (1975) Cyclic changes in volatile acidic metabolites of human vaginal secretions and their relation to ovulation. J.Chem. Ecol. 1., 361-376
Richmond, M., and Conoway, C.H., (1969). Induced ovulation and oestrus in Microtus ochrogashi. J. Reprod. Fertil. Suppl. 6, 357-376.
Siegel, S. (1956). Non-Parametric Statistics for the Behavioral Sciences, McGraw-Hill, New York.
Treloar, A.E., Boynton, R.E., Behn, D.G. and Brown, B.W. (1967). Variations of the human menstrual cycle through reproductive life. Int. J. Fertil. 12, 77-126.
Veith, J., Buck, M., Gertzlaf, S. Van Dolfsen, P. and Slade, A. (1983). Exposure to men influences the occurrence of ovulation in women. Physiol. Behav. 31, 313-315.
Vollman, R. F. (ed.), (1977). The Menstrual Cycle. Major Problems: Obstetrics and Gynecology, Vol. 7. Saunders, Philadelphia.
Whitten, W. K., Bronson, F.H. and Greenstein, J.A. (1968). Estrus-inducing pheromone of mice: Transport by movement of air. Science, 161, 584-585.