Sexual Behavior Frequency and Biphasic Ovulatory (Fertile) Type Menstrual Cycles

Winnifred B. Cutler 1,2, George Preti 2, George Huggins 1, Belle Erickson 1 and Celso Ramon Garcia 1
1. Department of Obstetrics and Gynecology, School of Medicine, Hospital of the University of Pennsylvania.
2. Monell Chemical Senses Center, Philadelphia, PA.
Physiology and Behavior, Vol. 34, pp. 805-810., 1985.
© Copyright 1985. Pergamon Press Ltd.
*College students whose menarche had occurred 7 years previously, prospectively recorded menstrual and sexual behavior history for 14 weeks as well as basal body temperatures. Regular weekly coital activity associates with the highest incidence of fertile type cycles in this sample of young women as follows:
2) Either of two heterosexual behaviors (coitus and/or genital stimulation by a man) were behaviors which were adequate as associates of 29.5+ 3 day cycles.
4) Women with regular weekly coital activity had the highest incidence (90%) of fertile-type basal body temperature (BBT) rhythms. Sporadically active women had the next highest incidence (55%) of fertile type BBT rhythms. Celibate women had the lowest incidence (44%) of fertile type BBT's
KEY WORDS: menstrual cycle length, fertility, sexual behavior, infertility, basal body temperature, celibacy
The length of a woman's menstrual cycle has long been known to bear some reflection of the underlying endocrine milieu as well as her potential for fertility. Vollman et al., as well as Treloar, et al. have, through their extensive prospective studies, clearly found that women whose cycles approach the 29 day span have the highest likelihood of a fertile cycle, while women whose cycles become longer or shorter have a proportionately diminishing incidence of fertile cycles (18, 20). With increasing age (up to about age 26) there is an increasing tendency toward 29 day cycles.
Recently, several other studies further demonstrated the relationship between reproductive behavior and fertility [1, 3, 4, 5, 6]. In 1979 our first prospective, double-blind study was published showing that women who had regular weekly, heterosexual activity had menstrual cycles of about 29 days, while women who either had a sporadic sexual activity or who were celibate tended to have a higher frequency of aberrant cycles (<25 or >33 days) [4]. Our initial study reported this phenomenon in four sub-samples of women whose data were prospectively gathered over different years and revealed its occurrence among undergraduate college women as well as within a sample of infertility patients [4,5, 6]. Further studies showed that luteal phase defects (shortened, hyperthermic phase) and sporadic sexual activity in the luteal phase were associated in a population of infertility patients [5]. Delayed age at first coitus was associated with a higher frequency of subsequent infertility as well [3,12].
More recently, Veith et al. have shown that women who 'slept with' men more than twice within a forty day span had a higher likelihood of having an ovulatory menstrual pattern than women who 'slept with ' men less often [19]. In their data of 40 women, 92% of those who slept with men two or more times in the 40 day span of the study showed ovulation according to analysis of basal body temperature graphs. In that study, presumptive ovulation was defined if a marked dip in temperature 13 to 15 days before the menses was followed by an increase of approximately 0.60F with luteal phase mean temperature being higher than the follicular phase mean. In contrast, only 44% showed presumptive ovulation among the group whose members slept with a man less often. While the authors reported no difference in ovulatory potential among women reporting coitus <2 days in 40 vs >2 days in 40, they did not report evaluation of weekly or other coital frequencies and ovulation. Our previous studies [1,4,5,6,] have established the value of “weekly” paradigms in sexual behavior/menstrual cyclicity studies and have further shown that the coital total is not a relevant measure which will correlate with cycle lengths [6]. Regular stable patterns of sexual behavior yield significant associations with cycle patterns; overall totals are irrelevant. Earlier, McClintock had shown that in addition to the fact that women who live together tend to cycle together, women who “saw men” three times a week or more tend to have menstrual cycles that were closer to 29 days than women who saw men less frequently [11]. In that study, the women were attendees at an all female college and, thus, their exposure to men had the potential of being quite limited.
Because of the relationship between menstrual cycle length and fertility [18,20] we have extended our studies of sexual behavior and menstrual cycle length to examine the relationship between sexual behavior, basal body temperature patterns and menstrual cycle length. We also attempted to determine which aspects of sexual behavior might be most informative as an associate of underlying fertile cyclicity.
The present study evaluated whether self-stimulation (masturbation), coitus, and/or heterosexual genital stimulation by a man without coitus, could be differentiated in terms of their relative association to fertility. Presumptive fertility (P. fertility) was evaluate two ways: by the menstrual cycle length [4] and by analysis of basal body temperature chart [5]. These data demonstrate relationships between sexual behavior and P. fertility as reflected by basal body temperature patterns and menstrual cycle length.
METHOD
The study was designed as a prospective double-blind data collection and analysis. Research assistants were recruited through an ad in the University newspaper which offered students and opportunity to participate in research about sexual behavior and fertility. Fourteen women students who were qualified and accepted as research assistants met with one of us (WC) at least every fourteen days throughout the spring semester of 1983 at the University of Pennsylvania. At each meeting, further instructions about research design and the role of each student within the study was presented.
Human study committee approvals and informed consent were obtained before enrolling subjects. In the first week of the study, the fourteen research assistants recruited 194 undergraduate students by soliciting at randomly assigned dormitory locations on campus. All met the following criteria: “gynecological maturity” as defined by Treloar et al. as menstruating at least seven years, nulliparous, unmarried, not a freshman nor a final-semester senior, not currently (nor within the last three months) using oral contraceptives nor an IUD, and a willingness to make a daily entry of basal body temperature and menstrual and sexual behavior.
All qualifying subjects were specifically invited with assurances that “normal” as well as “abnormal” patterns of cycling are important to this research. Each subject was provided a research packet containing instructions, basal body temperature chart, basal body temperature thermometer and menstrual/sexual calendar card. She selected her own code name and did not reveal this to her research assistant. The subjects were told that they were participating in a study designed to increase knowledge about the reproductive biology of women. Neither the research subjects nor the research assistants knew the specific purpose of this study.
Regular follow-ups were scheduled, subjects were routinely contacted approximately every three weeks during the semester for the purposes of stimulating continuation and maintaining accurate reporting. Throughout the semester, questionnaires were completed that gathered data on tobacco and drug use, deaths in the family, medical history, academic load, life-style stresses, exercise habits and socio-economic circumstances in an attempt to dilute the emphasis on the real purpose of the study. At the end of the semester, a closing survey was completed among which were questions requesting the subject to score won a scale of 1 to 5 how accurately she had recorded her sexual, menstrual and basal body temperature patterns. These questions were included as a validation procedure to allow the removal of subjects who might have skipped days and later backfilled.
In order to reassure the subjects of their anonymity, and maintain a double-blind research design, a specific procedure was followed. At the end of the fourteen week study, each research assistant met with each subject, and without viewing the data, had the subject seal the data in an unmarked envelope. The packet was then mailed to one of the authors (WC).
Each basal body chart was scored in double-blind fashion by one of us (BE) and checked by another (WC). The chart was categorized as: presumed normal ovulatory (+), presumed ovulatory with a subsequent inadequate (too short) hyperthermic phase, i.e., 12 days (-I), or anovulatory (-) by the method described in an earlier study [5]. In order to be presumed normal ovulatory (+), the cycle had to meet the following criteria: a thermal nadir in basal body temperature at least 12 days before the next menses, which was followed by a rise in temperature that was sustained throughout at least the last 12 premenstrual days. To achieve a score of presumed ovulatory with an inadequate hyperthermic phase (-I), the graph had to show a preovluatory nadir that was followed by a rise which was sustained, but which remained elevated for less than 12 days. Luteal phase (postovulation) lengths shorter than 12 days are considered to reflect an inadequate luteal phase (for a fuller discussion and literature review, see [5]). A score of anovulatory (-) was assigned to those basal body temperature charts which had no midcycle nadir followed by a rise which was sustained, i.e. monophasic.
Data Analysis
One hundred and twenty subjects completed the study, mailing back documents to the Monell Chemical Senses Center. Of these, 26 were disqualified for a variety of reason including: missing data, certain prescription drugs or use of contraband substances such as marijuana. This left a remainder of 94 who completed all requirements, including a self-report showing a high level of compliance with the prescribed requirements of participation (i.e., no backfilling or omission of prospectively collected data). Eighty-three of these completed sufficient temperature records to permit BBT evaluation; eleven left too many blank days to define a BBT pattern.
Figure 1. One notes that the Weekly Group had a predominance of women with cycle lengths in the stippled (fertile type)range of 29.5+ days. There were NO short cycles among the women with Weekly Behavior. Women with sporadic and celibate patterns of coital activity showed a weide range of short, normal, and long cycles.
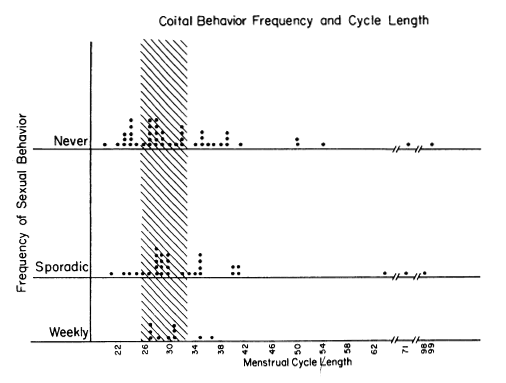
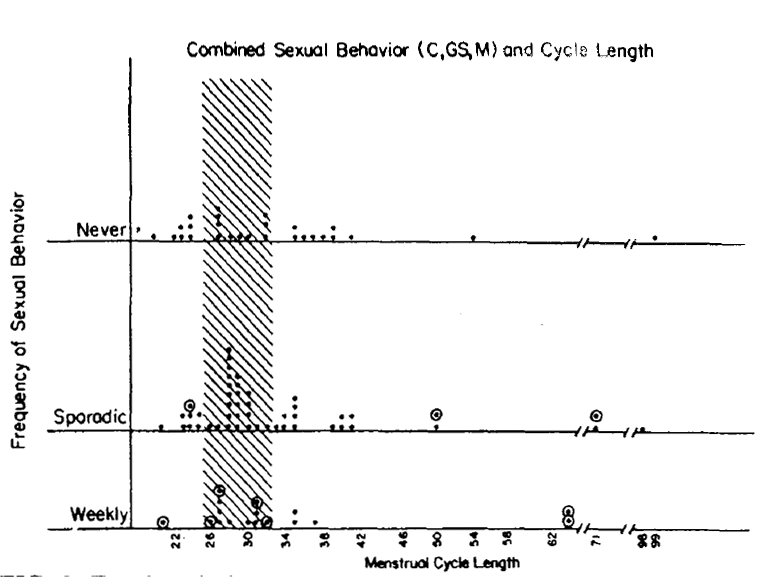
Figure 2. Each symbol represents data of one subject. No subject is represented more than once. The data which are outlined (o) reflect subjects who moved (See Fig. 1) from a lower frequency class to a more frequent behavior class by virtue of including genital stimulation by a man as a new criteria for behavior. Thus, for example, among the weekly active group, 2 women showed 35 day cycle: the one which is outlined (o) represents a woman who had failed to acheive a weekly score until genital stimulation was added to the behavioral criteria.
Figure 3. For description see Fig. 2. One notes that when masturbation is considered as an adequate criterion for "behavior", weekly sexual behavior fails to associate with 29.5 + 3 day cycles. Thus, for example, among the weekly group, the one 21 day cycle point outlined (o)indicates a woman who became weekly by virtue of permitting masutbation as a criterion for secual behavior. In contrast to self stimulation behavior, heterosexual behaviors (Figs. 1 and 2) that acheive a weekly frequency show NO very short or very long menstrual cycle lengths.
Statistics
Tabled data were analyzed for significance by the Chi Square Test. Analysis of Variance statistics were inappropriate because the data are not parametrically distributed (see Figs. 1, 2, and 3). Accordingly the Segal-Tukey test [9] was chosen to ask: “How varied are the array of scores in one sample of people vs. the other ?”
A subject's sex behavior was recorded in the fooling manner. If she has sex at least once in each 7 day non-menstruating week her behavior was scored “weekly”. If she did not have sexual behavior each and every week but did record behavior during the course of the study, she was scored “Sporadic.” If she did not record any sexual behavior, she was scored “Never.” Several different sexual behaviors were evaluated: intercourse, genital stimulation by a man, and masturbation. Weekly sexual behavior defined the experimental condition; less than weekly (Sporadic and Never) defined the control condition and cycle length was the variable being tested (”score”). “Weekly” categorization required a report of sexual behavior every non-menstruating week of the term and thus reflected an ongoing pattern of weekly behavior that had been prospectively recorded. A rank ordering of scores from the extremes towards the median permitted one to test whether different conditions showed a different range of cycle lengths. One was therefore measuring relative variance of scores from the two conditions. In analyzing menstrual cycle data when multiple scores are available fore each subject, statistical rigor requires that one score per subject be selected [4]. The first cycle of each subject was selected for analysis because: (1) there was no adequate way of dealing with different numbers of cycles per subject since subjects had one, two, there or four menses onsets during the span of the study; and (2) observations from any one individual have a degree of dependency. The first cycle was selected for all figures but previous studies have shown that regardless of whether one tests first, last, or mean cycle lengths the results tend to be very similar [4].
RESULTS
Figure 1 shows the coital and menstrual frequencies. Each dot represents the data of one subject and each subject is recorded only once in any figure. The pattern in 1983 is similar to the pattern obtained in our earlier study [4]. Women who maintained a weekly coital frequency through the semester had no incidence of short cycles and no very long cycles. The stippled area of the graph outlines the 29.5+ 3 day range, which has the highest likelihood of having ovulatory basal body temperature rhythms [20]. Statistical analysis, by the Segal-Tukey test which accommodates to differences in group size [9], revealed a statistically significant difference in cycle lengths between sexual behavior groups (weekly vs. less than weekly: z=2.03, p<0.04).
Figure 2 analyzes the same group of women considering all heterosexual behavior ( coitus and/or genital stimulation by a man) as adequate criteria for sexual behavior.
Figure 2 also shows that women who have weekly heterosexual behavior, whether the behavior is defined as coitus, or genital stimulation, or any combination of both show an absence of short cycles and a lower incidence of long cycles than women who have less frequent heterosexual activity. This result is statistically significant (z=2.06, p<0.04). This replicates the 1979 results [4]. The figure also indicates, by the outlined circles around the relevant data points, which women moved into higher frequency categories by virtue of the inclusion of genital stimulation as an added criteria for sexual behavior.
The question of whether masturbation might be an adequate behavior for revealing a similar association between weekly sexual behavior and fertile-type menstrual cycle lengths was also addressed in this study. There were insufficient numbers of women to analyze masturbation as a separate entity. Masturbation could be analyzed in combination with heterosexual behaviors. When we do permit masturbation to be called “sexual behavior, “ then weekly sexual behavior fails to associate with fertile-type menstrual cycles. Figure 3 shows the combined sexual behavior (masturbation or coitus or genital stimulation by a man in any combination)) of each woman by cycle length. When this combined sexual behavior pattern is analyzed, one finds that “weekly” frequency of sexual behavior is no longer an adequate associate of normal type (29.5+ 3 day) lengths. Thus, there was a failure to achieve statistical significance (z =0.71, p<0.48). In the 1979 study, masturbation appeared to be an inadequate associate of cycle length but a small N limited any definite conclusion. With both studies showing the same thing a stronger conclusion is reasonable [4]. Consequently, self-stimulation does not appear to be a biologically adequate substitute for heterosexual behavior.
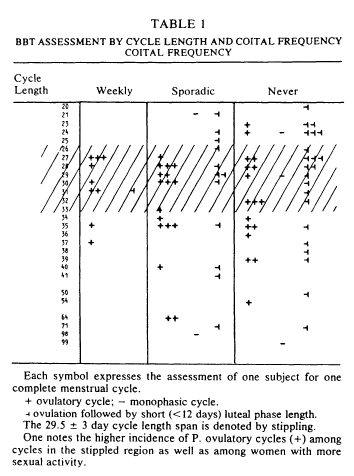
Basal body temperature charts were analyzed next. Table 1 arrays the basal body temperature assessment by cycle lengths and coital frequency for all 83 subjects. Within the weekly category, all but one (90%) of the subjects had a presumptively normal ovulatory basal body temperature rhythm. One case was apparently ovulatory with a short luteal phase. In contrast, the data of the 31 women who were sporadically active revealed a lower (55%) incidence of P. fertile cycle patterns. Of the 43% who did not show a P. fertile cycle, 12 out of 14 showed evidence of ovulation but ovulation with short luteal phase. The celibate women were somewhat similar to the sporadic women in pattern of basal body temperature rhythm. Again, the most common deficit was short luteal phase.
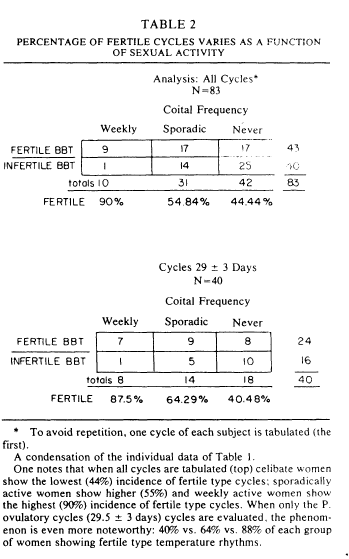
Table 2 arrays the percentage of women showing P.fertile cycles as a function of sexual activity pattern. The table is broken into two parts. The top shows the analysis for all cycles. Among the 83 women who supplied complete data, one notes that ninety percent of the weekly active women were P. fertile in basal body temperature rhythm, 55% of the sporadically active women showed a P. fertile basal body temperature rhythm, and 44% of the celibate women showed a fertile basal body temperature rhythm. Perhaps more interesting is the pattern revealed when one looks at the 29+3 day cycle lengths, those which have a high likelihood of ovulation [20]. One finds that celibate women with circa 29 day cycles have the lowest (40%) incidence of P. fertile basal body temperature rhythm. As table 1 shows, the most common problem is not the absence of ovulation, it is a short postovulatory phase length, a situation which is commonly found in a luteal phase defect. Analyses of the data in Table 2 provides statistical support: for all cycles, x2(2)=6.2, p<0.2; for 29+3 day cycles, x2(2)=4, p<0.025.
DISCUSSION
Weekly heterosexual sexual behavior is consistently associated with menstrual cycle lengths of 29+3 days, both in this study and the former ones [4,5,6]. Self-stimulation behavior did not show a similar association between weekly behavior and circa 29 day menstrual cycle lengths. This is consistent with a prior study [4].
These data show that women who report regular weekly sex with men have a greater incidence of presumptively fertile menstrual cycles than those who report less sexual activity. In this study, there were much fewer numbers of women with regular weekly sexual activity because oral contraceptive or IUD use disqualified women from participating. We suspect that most women who are involved in a stable sexual relationship are contracepting with either the pill or the IUD. The greatest cause of withdrawal from the study is attributed to the inception of these forms of contraception's as well. It should be pointed out that the disproportionate seizes of the different groups are adequately factored into the non-parametric tests used [9]. However, despite the disproportionate group sizes, the differences between them reach statistical significance.
Moreover, the chief problem associated with less frequent sexual activity, in all of our studies points less to a failure of ovulation but rather to a more subtle problem - the short luteal phase. A fertile menstrual cycle requires both an ovulation and an adequately long luteal phase to ensure sufficient time and steroid output to prepare the endometrium for implantation (for a fuller discussion see [5]).
The data, presented above, show that sexually active women who have regular, stable patterns of sexual activity have significantly more fertile cycles than women of the same age who have sporadic patterns of sexual activity. Sexual activity with men is distinguished from sexual activity of a masturbatory nature in this study as well as in our former study. Consequently, the normalization (i.e, approach toward 29 day cycle length) of the menstrual cycle is due to something more than genital stimulation and the presence of a male partner appears to be one of the critical factors in the cycle normalization process. Further support of our finding can be found in the data gathered by McClintock [11]. In this study, women who “saw” males less that 3 times/week had cycles which moved away from the normal cycle length of 29.5 days. A more recent study [15] showed a similar trend. Women were grouped according to how many times per week they “saw” men and those who “ saw” men more often had menstrual cycles whose mean length more closely approached the 29.5 day average. Women seeing men most frequently in that study (e.g, 5 times/week) had an average cycle length of 29.45 days. Of interest in the analogy these results have to studies and observations conducted with non-human primates and other mammals [7,8,10,14,16,17,21]. In these studies, the presence of a male or his odors is important in regulating cyclicity and/or fertility [7,17].
The possibility that odors play in human reproductive biology has received considerable recent discussion particularly with respect to female-female interactions (i.e. menstrual synchrony). While discussions of male-female interactions have often centered on the possibility of a releaser type effect (i.e, and 'aphrodisiac'), little consideration of male-female primer type interactions can be found in scientific or lay literature. A regulation of the female cycle which is mediated, in part, by the odors of a male partner is one possible explanation of the phenomenon reported here.
These results can be considered from a biological development perspective. It is known that young women pass through a state of relative infertility that by about age 26 has developed into a fertile endocrine system for most [2,13,18]. It is also clear that some women reach this fertile pattern earlier than others. We suggest that the onset of regular sexual activity is one of the components which help to develop the reproductive endocrine system and that human sexual activity could be understood to be important to the endocrine state of an individual woman.
These data support the idea that relationship exists between heterosexual activity and menstrual cycle normality as well as between heterosexual activity and fertility. Although the direction of causality between the physiology-behavior association cannot be proven, the following can be seen in the data: (1) regular heterosexual behavior predicts a 29+3 day cycle but the 29+3day cycle doesn't predict regular heterosexual behavior (see Figs. 1 and 2 where “Sporadic” and “Never” subjects have 29+3 day cycles); and (2) if the physiology defined the course of behavior we would expect to see weekly sex when we see 29+3day cycles, which in the absence of a partner would be reflected in regular masturbation. However, as noted above, 29+3 day cycles do not produce regular masturbation or weekly heterosexual behavior in these studies. Therefore, behavior appears to alter physiology. In the case of infertility it would appear that couples who are trying to conceive, should engage in sexual activity at least once in every non-menstruating week, to better sustain the luteal phase of the cycle and preferably twice a week to assure adequate insemination.
ACKNOWLEDGEMENT
REFERENCES
1. Cutler, W.B., J.M. Davidson, and N. McCoy. Relationships between estrogen level, hot flashes and sexual behavior in perimenopausal women. Neuroendocrinol. Lett 5:185, 1983.
2. Cutler, W.B. and C.R. Garcia. The psychoneuroendocrinology of the ovulatory cycle of women. Psychoneuroendocrinology 5: 89-111, 1980.
3. Cutler, W.B., C.R. Garcia and A. Krieger. Infertility and age at first coitus: A possible association. J Biosoc Sci 11:425-432, 1979.
4. Cutler, W.B., C.R. Garcia and A. Krieger. Sexual behavior frequency and menstrual cycle length in mature premenopausal women. Psychoneuroendocrinology 4: 297-309, 1979.
5. Cutler, W.B., C.R. Garcia and A.M. Krieger. Luteal phase defects: A possible relationship between short hyperthermic phase and sporadic sexual behavior in women. Horm Behav 13: 214-218, 1979.
6. Cutler, W.B., C.R. Garcia and A.M. Krieger. Sporadic sexual behavior and menstrual cycle length in women. Horm Behav 14: 163-172, 1980.
7. Howard-Tripp, M.E. and C. Bielert. Social contact influences on the menstrual cycle of the female chacma baboon (Papio ursinus). J S Afr Vet Assoc 49: 191-192-1978.
8. Johns, M.A. Reflex ovulation in light-induced persistent-estrus rats: Role of the vomeronasal system, the adrenal and ovarian steroids. Unpublished Ph.D. Thesis, Rutgers University, Newark, NJ. 1979.
9. Lehman, E.L. Nonparametrics Statistical Methods Based on Ranks. San Francisco: Holden-Day, 1975.
10. Marsden, H.M, and F. H. Bronson. Estrous synchrony in mice: Alteration by exposure to male urine. Science 144:1469, 1964.
11. McClintock, M. Menstrual synchrony and suppression. Nature 229:244-245, 1971.
12. McFalls, J.A., Jr. Impact of VD on the fertility of the U.S. black population. Soc Biol March: 2-19, 1973.
13. Metcalf, M.G. and J. A. Mackenzie. Incidences of ovulation in young women. J Biol Sci 12: 345-352, 1970.
14. Perachio, A.A. Hypothalamic regulation of behavioral and sexual performance. In: Recent Advances in Primatology, vol 1, edited by D.J. Chivers and J. Herbert, New York: Academic Press, 1978. P. 549
15. Quadagno, D.E. , H.E. Shubeita, J. Deck and D. Francouer. Influence of male social contacts, exercise and all-female living conditions on the menstrual cycle. Psychoneuroendocrinology. 6: 239-244, 1981.
16. Richmond, M. and C.H. Conaway. Induced ovulation and oestrus in Microtus ochrogaster. J Reprod Fertil (Suppl) 6: 357-376, 1969.
17. Schwartz, N.B. Mechanisms controlling ovulation in small mammals. In: Handbook of Physiology: Endocrinology II. Part 1. Baltimore: Waverly Press, 1973. Pp. 125-141.
18. Treloar, A.E., R.E. Boynton, D.G. Behn and B. W. Brown. Variation of the human menstrual cycle through reproduction life. I J Fertility 12: 77-126, 1967.
19. Veith, J., M. Buck, S. Getzlaf, P. Van Dalfsen and A. Slade. Exposure to men influences the occurrence of ovulation in women. Physiol Behav 31: 313-315, 1983.
20. Vollman, R.F. The menstrual cycle, vol 7. In: Major Problems in Obstetrics and Gynecology. Philadelphia: W.B. Saunders, 1977.
21. Whitten, W. K., F.H. Bronson and J.A. Greenstein. Estrus-inducing pheromone of male mice: Transport by movement of air. Science 161: 584-585, 1968.