Stress Urinary Incontinence:
A Pervasive Problem Among Healthy Women
Published in the Journal of Women's Health, (Vol 1. Number 4, 1992)
Copyright ©1992. Mary Ann Liebert, Inc., Publishers
Stress Urinary Incontinence:
A Pervasive Problem Among Healthy Women
WINNIFRED B. CUTLER, Ph.D., ERIKA FRIEDMANN, Ph.D., KATE FELMET, and ELIZABETH GENOVESE, M.D.
Abstract:
"Trouble with involuntary loss of urine” is acknowledged by more than one half of the 194 women in two health conscious mostly well-educated populations of presumably healthy women. The proportion of women experiencing incontinence remains relatively constant but the severity increases with age. Multiparity, poor pelvic muscle strength or endurance, and postmenopausal status are the most common associates of stress urinary incontinence (S.U.I.) in an otherwise healthy woman.
We recommend that clinicians and other investigators be alert to screening for S.U.I. Because the literature shows that biofeedback retraining of the pelvic floor muscles can improve poor pelvic muscle strength and endurance and simultaneously reduce the frequency and severity of incontinence, the search for women who might benefit from this information is particularly relevant.
Introduction
Stress urinary incontinence (S.U.I.) in women has been addressed in the literature under specific circumstances of its exacerbation (pregnancy, parturition and old age) or when outcomes of treatment are reviewed after surgery or pelvic muscle training.1-12 Among women with incontinence, pelvic muscle strength is closely related to the incidence, severity, and recovery from incontinence. According to Kegel’s earliest and most extensive examination of S.U.I., the woman’s failure to develop what he termed “a normal action pattern” in early life was commonly associated with S.U.I.1
In studies of approximately 500 women with confirmed stress urinary incontinence, pubococcygeal muscle function loss was present in every woman.2 We could find no studies spanning the active adult years (20-64) that evaluated the incidence of S.U.I. among healthy women in relation to physiologic measures of pelvic floor muscle strength or endurance.
All studies of incontinence evaluated those who were already incontinent; except where the impact of pregnancy on incontinence was specifically being evaluated.1-12
It was appropriate to determine the incidence of S.U.I. among healthy women and its relationship to selected variables because the data had not previously been reported.
Materials and Methods
We used data from two separate samples gathered 11 years apart. They were in 1980, in the Stanford Menopause Study and in 1990-1991, in the Athena Wellness population.
The populations studied:
In 1979 the Stanford Menopause Study was established (by the senior author of this article with Dr. Julian Davidson) to longitudinally enroll and then prospectively follow a cohort of healthy perimenopausal women. Characteristics of the Stanford sample have been previously described in papers reviewing relationships between their sexual habits, hormones and menstrual changes.3-15
These subjects of mean age 48.67± 0.34 (SEM) years, and premenopausal symptoms of hot flashes, night sweats, and menstrual cycle habit changes comprise a group about to enter menopause. Data were gathered at several consecutive interviews every 7 to 12 weeks during the first year, and with waning frequency thereafter. Using both written reports and contact interviews, we gathered information to study the nature of the perimenopausal transition symptom patterns in an initial sample of 153 presumably healthy women who were not taking hormones.
In the first two interviews, subjects were interviewed by a trained perimenopausal contemporary in a mutually convenient location using questionnaires that provide space and time to mention concerns. Because of what seemed a frequent mention of a concern with incontinence, a specific question was included for those who proceeded to the third interview. The third interview required subjects to come to Stanford for an interview with the senior author, and for plasma sampling.
Geographic constraints and an apparent squeamishness about phlebotomy reduced the sample size to 57 women at the third interview. The question asked of these 57 was: “Loss of urine sometimes occurs involuntarily. Does this every happen to you? Under what situations? How often?” The second population allowed us to gather more data among a wider age range.
The Athena Institute for Women’s Wellness Research was established by the senior author in 1986. The first physician-run Athena Wellness program opened in February 1990, examining healthy women who were participating in an “executive level” comprehensive physical examination. Because the price for this 2 1/2 hour wellness evaluation exceeds $300.00, the population is either composed of corporation-paid management-executive level women, or those choosing the exam independently. Thus, the women tended to be of high socioeconomic status.
When the wellness program opened, pelvic muscle testing using a programmed perineometry system was available as an extra-cost option. Among the first 80 patients, 43 (54%) chose to undergo this test. Effective with patient 81, the pricing policy was changed to include pelvic muscle testing in the package price of the executive exam. Among the 60 subsequent patients enrolled, 57 (95%) underwent this testing.
By 1991, the Athena Wellness population included 140 women; simple incontinence data were available for 137. Every individual was asked to indicate if she had “trouble with involuntary loss of urine.” Because it had become apparent that many young women also were indicating a problem with incontinence, we subsequently developed a detailed questionnaire for the latter 60 women. This included information about severity and frequency of stress urinary incontinence, nocturia, minipad use and fluid consumption.
Nocturia was examined because of its potential to parallel an already-recognized, age-related alteration in physiology. It was known that hot flashes are a major early symptom of hormonally related perimenopausal symptoms and the association had recently been made between nocturnal disturbances in thermoregulation (night sweats) and daytime flashes.16 We thought it reasonable to address a similar question about urinary system stresses.
Pelvic muscle testing was conducted with an electromyographic (EMG) perineometer (PerryMeter system, Strafford, PA) in 100 of the 137 women for whom data are presented: for 43 of the first 80 (without) extensive incontinence questionnaire, and for 57 of the final 60 (with) extensive incontinence questionnaire. The time of day that the perineometry measurement was taken was also noted.
Perineometry
EMG perineometry assesses the strength and the tone of the muscles of the pelvic floor including sphincters. The technician guides the patient through a computer-timed standard protocol including both quick (“flick”) and 10-second (“hold”) pelvic muscle contractions.17 Holding the muscle at maximum tension for 10 seconds produces a calculated digitized mean “hold” score. Six consecutive “holds” are alternated with 6 consecutive ”relax” intervals.
Pelvic muscle strength is calculated by subtracting the resting muscle tension (the calculated average of each of the 6 “relax” scores) from the average score obtained with 6 consecutive holds to form the net muscle strength score. The measures provide data of tonic strength; that is, the calculated, digitized, mean 10 second “hold” score. The phasic strength is calculated by averaging the 6 consecutive “flicks”.
Reliability of the equipment was tested with the test-retest method using the second and third consecutive “hold” scores. The endurance score reflects the number of seconds a woman can hold muscular tonus at or about 50% of her own maximum “flick” strength.
Questionnaire data
Questionnaires were completed in private by filling in a preprinted form. The questionnaire included data analyzed for question pertaining to the following concerns: S.U.I. prevalence: age relation ship to S.U.I.; minipad use frequency; whether parity affects the prevalence of S.U.I.; nocturia incidence and age; whether poor pelvic muscle strength places women at risk for S.U.I.; and, using perineometry, assessing whether pelvic muscle strength is lower in the afternoon than in the morning as per Kegel’s suggestion.
Statistical testing
Statistical testing included Chi-Square tests, t-tests, analysis of variance, and Pearson’s correlation coefficient.
Frequencies of poor pelvic muscle strength and incontinence, parity and incontinence, and the relative frequencies of incontinence across the age categories were examined with Chi-square. Differences in average pelvic muscle strength and endurance between incontinent and nonincontinent women were evaluated with t-tests. Analysis of variance was used to evaluate the effect of time of day on perineometry scores. In this analysis, time was a 3 level grouping variable and perineometry scores were the dependent variable.
Reliability testing
Pearson’s correlation coefficient was used to evaluate the reliability of perineometric recording using two successive measures within each patient. Figure 1 shows the distribution of these scores. (r = .9606, P < .0001, r2 = .9227)
FIG. 1. Perineometric strength: two measures.
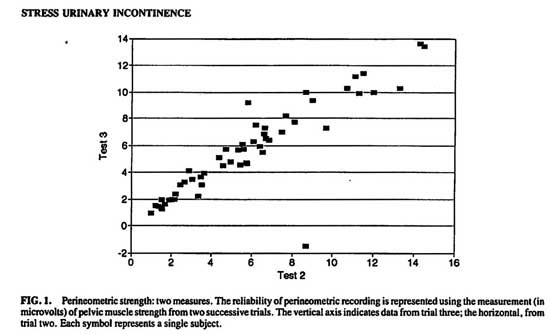
The reliability of perineometric recording is represented using the measurement (in microvolts) of pelvic muscle strength from two successive trials. The vertical axis indicates data from trial three; the horizontal, from trial two. Each symbol represents a single subject.
Results
Incidence of urinary incontinence
S.U.I. is acknowledged by 106 of 194 women studied. In the Stanford Menopause Study, 60% of 57 premenopausal women responded “yes” to the presence of involuntary loss of urinary control at times. All other results that follow refer to the subsequent 1990-1991 Athena Wellness sample.
In the Athena Wellness population, 52% of the sample of 137 women acknowledged “trouble with involuntary loss of urine”. The frequency of reporting incontinence was not related to age. [Chi-square (8, n = 137) = 3.75, P > .2 for 5-year intervals. See Figure 2.] Further groupings at age intervals 20-34, 35-49, 50-65 confirmed this lack of age effect. [Chi-square (2, n = 137) = 1.22, P > .2]
The more detailed data about severity and consequences of S.U.I. were available for 60 patients. Among these 60 women, 15 (25%) said they never have an involuntary loss of urine; 26% say it occurs on rare occasions, 34% say it occasionally occurs and 10% have it often. (See Fig. 3) Until age 32, none of the 60 women reported any experience with S.U.I. Until the age of 39, none reported more than mild S.U.I., and after the age of 39 the incidence of more severe incontinence balloons.
The use of minipad to confine urinary leakage is reported by 30%. And 22% of the entire sample acknowledge the use of minipads occasionally.
Multiparity is associated with a high incidence of S.U.I. Table 1 shows that 84% of multiparous women report a problem with S.U. I. vs. 63% of nulliparous and primiparous women. The difference was statistically significant as well as substantial [Chi-square, (1, n = 59) = 3.46, P < .05]
The raw data showed that 65% of the nulliparous group and 57% of the primiparous group acknowledged S.U.I. The distribution of our sample size did not permit us to separately test for a difference in incontinence between the 20 nulliparous and seven primiparous women because of small expected values in several of the cells. We were able to test, in a 3 X 2 Chi-square, the distribution of nulli, primi, and multiparity vs. presence or absence of incontinence. These results also showed that multiparous women report a higher prevalence of incontinence [Chi-square, (2, n = 59) = 5.77, P < .05].
Pelvic muscle strength
Pelvic muscle strength is poor among incontinent woman. (See Table 2) When EMG perineometrically measured pelvic muscle strength was < 5.7 mv or endurance was < 5 seconds, the score was considered “poor”. Women who have a poor score have a much higher incidence of incontinence than those with better scores (56% vs 29%). Conversely, 77% of the incontinent women have poor pelvic muscle strength or endurance vs 52% for continent women. This finding was also statistically significant. [Chi-square, (1, n = 60) = 4.8, P < .025].
Pelvic muscle strength was lower among women tested in the afternoon than among women tested in the morning or the evening. Using the analysis of variance, the data revealed significant difference among the three time periods: morning (n = 44, mean, 6.075 mv, S.E.M. = .4874) afternoon (n = 35, mean, 4.02 mv, S.E.M. = .4582), and evening [n = 16, mean, 7.23 mv, S.E.M. = .7614; (F2,92) = 7.64, P < .001)]. Pelvic muscle strength in morning was higher than in the afternoon [(F 1,92) = 9.07, P = .0034].
FIG. 2. Relative ratio of incontinence by age.
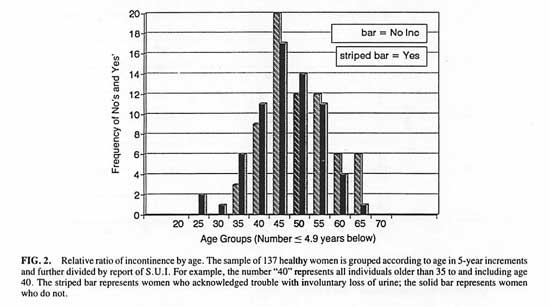
The sample of 137 healthy women is grouped according to age in 5-year increments and further divided by report of S.U.I. For example, the number “40” represents all individuals older than 35 to and including age 40. The striped bar represents women who acknowledged trouble with involuntary loss of urine; the solid bar represents women who do not.
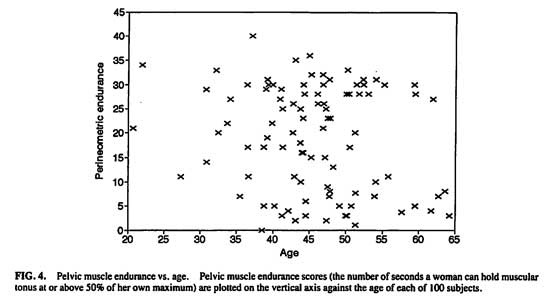
Potential age effects on pelvic muscle strength and endurance were examined. (Figs. 4 and 5) The endurance score reflects the number of seconds a woman can hold muscular tonus at or above 50% of her own maximum phasic (“flick”) strength. There was no direct linear relationship between age and either strength (r = -.0202, P > .8) or endurance (r = -.0041, P > .8).
Grouping the women into pre-age 50 vs. post age 50 was tested next. Women 50 and above had lower pelvic muscle strength (n = 28, mean, 4.32 mv, S.E.M. = .51) than younger women [n = 69, mean, 7.38 mv, S.E.M. = 1.43; t(82.91df) = 2.01, P < .05]. There was no difference in endurance between the older (mean, 19.6 seconds, S.E.M. = 2.03) and younger women [mean, 19.1 seconds, S.E.M. = 1.30; t(95df) = -.21, P < .85].
Nocturia
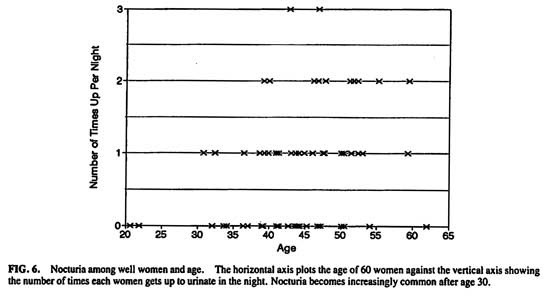
Nocturia becomes an increasingly common event after the age of 30. (Fig. 6) Significantly fewer women younger than age 45 (49%) get up at night than after age 45 (71%) [Chi-square (1, n = 60) = 3.72, P < .05].
FIG. 3 Stress incontinence severity. The height of each bar indicates the number of subjects with a given severity of incontinence, where “0” indicates never and “4” indicates always. Twenty-five percent of these 60 women report “never”
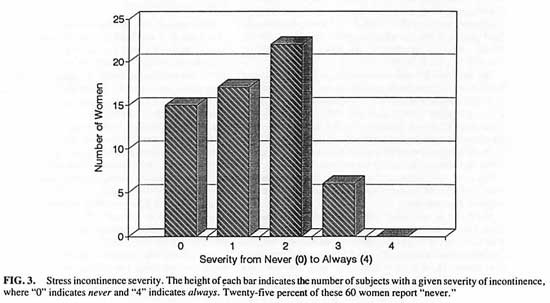
Table 1. Multiparity increases the Incidence of Incontinence (Chi-Square = 3.46 P < 0.5)
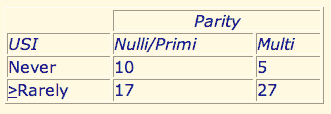
*Frequency of involuntary loss of urine is shown relative to parity in 59 women; 84% of multiparous women report that they > rarely experience SUI compared with 64% of nulli- and primiparous women.
Hormonal replacement therapy (HRT)
Fifteen women (of 100 tested) were currently using HRT but the variation in timing of onset ranged from several years to several weeks. Likewise, dosages of regimens were varied. The fifteen who “currently used” HRT showed no higher level of perineometric strength or endurance than those of the same menopausal age not taking hormones
Comment
Trouble with involuntary loss of urine is acknowledged by more than half the women in a health-conscious, mostly well-educated population of presumably healthy women. The proportion of women experiencing incontinence remains relatively constant but the severity increases with age. Similarly, the incidence and frequency of nocturia increase with age. Pelvic muscle strength drops by age 50. Multiparity, poor pelvic muscle strength or endurance, and postmenopausal status are the most common associates of S.U.I. in an otherwise healthy woman.
Although only recently studied in “well” populations before menopause, the incidence of S.U.I. is clearly large enough to support investment in products and services attempting to meet women’s needs; surgery, diapers, drugs, and other therapeutic modalities.
When the Stanford Menopause Study was designed in 1978/79, involuntary urine loss had not been identified among the cluster of symptoms of either the menopause or perimenopausal transition. The classic “Kupperman Menopausal Index” was described in JAMA on contemporary therapy of the menopausal syndrome in 1959. Kupperman had identified a cluster of symptoms characteristic of the menopause that were responsive to estrogen replacement therapy. These symptoms included: vasomotor instability, parethesia, insomnia, nervousness, melancholia, vertigo, weakness, arthralgia and myalgia, headache, palpitation, and formication.18 Conspicuously absent was S.U.I.
Table 2. Perineometry Scores are Poor Among Incontinent Women (Chi-Square = 4.8 P = < .025)a
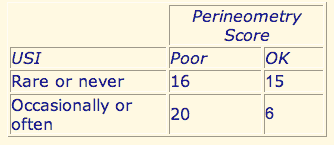
Urinary incontinence is compared to the perineometric pelvic muscle strength of 57 women. Fifty-nine percent of women whose strength is poor (tonic strength was < 5.7 microvolts of endurance was < 5 seconds) report S.U.I. compared to 29% of women with better EMG scores.
Within our first 6 months the Athena data showed that involuntary loss of urine was unexpectedly pervasive among women after age 30. Because a Medline search in mid 1990 revealed that no prospective data in healthy women had been published, we subsequently developed a detailed stress incontinence questionnaire that was provided to every woman entering the Athena Wellness Program after December, 1990. We closed the database in June 1991. (These data, in part, were presented at the North American Menopause Society’s second annual meeting in September, 1991.)
In May 1990, Nygaard et al. reported on exercise habits and incontinence prevalence in 326 gynecologic patients spanning a similar age range to ours.19 In their sample, 47% acknowledged S.U.I. – a figure that is comparable to our 52% prevalence rate.
Our cross sectional finding of significantly lower pelvic muscle strength in the afternoon supports Kegel’s untested perception. This result appears to differ from a 1991 report by Dougherty et al. that found no variation in time of day in their repeat measurements of “flick” strength of the circumvaginal muscles.20
However, the Dougherty study is not directly comparable to ours or Kegel’s because they tested only 5 subjects of widely divergent ages, at 4 specific times of day, and used a peak “flick” score that reflects the aberrantly highest score an individual can momentarily achieve. A “flick” score does not reflect tonic muscle strength. It is the tonic strength that showed a relationship to incontinence. The four times they chose may not reveal a cyclic variation in strength if it were occurring in the other, untested hours. Our own finding of an afternoon dip in scores, followed by an evening return to equivalently high levels of the morning may suggest something comparable to the afternoon slump in energy so commonly experienced by many adults.
Future investigators should use repeated measures, as in the Dougherty study, but focus on strength (“holds”) rather than “flicks” and test at more hours in order to test for a circadian cycle.
Long-term hormonal replacement therapy use was rare in the Athena Wellness population. Some women had recently begun hormonal therapy but very few were taking it at the time of testing. The 15 women who “currently use” hormone replacement therapy (HRT), showed no higher level of perineometric strength than those of the same menopausal age not taking hormones, but small samples, (and variation in timing of HRT) render these data inconclusive.
In 1951 Arnold Kegel, M.D. provided the first objective data (in JAMA) describing a highly effective physiologic therapy for S.U.I.1 In that classic paper, he demonstrated that the impaired status of the neuromuscular structure could only rarely be traced to congenital anomalies or disease of the spinal cord.
Kegel created a method, “vaginal perineometry,” for testing pelvic muscle strength using a device, the “Kegel perineometer” that he invented. It consisted of an air cone that was inserted vaginally and connected to a pressure sensitive transducer. Women without S.U.I. who had normal muscle tone, upon contraction of their vaginal muscles recorded scores between 30 and 60 mm Hg on Kegel’s perineometer. In contrast, women with S.U.I. showed either poor or absent muscular function; readings of 0 to 5 mm Hg were common.
FIG. 4. Pelvic muscle endurance vs. age. Pelvic muscle endurance scores (the number of seconds a woman can hold muscular tonus at or about 50% of her own maximum) are plotted on the vertical axis against the age of each of 100 subjects.
Kegel subsequently used the perineometer to therapeutically provide “real-time” biofeedback of contractile force of the muscles as patients generated contractions. He suggested that early childhood toilet training or some other early behavior had caused a lack of normal development of self control over the muscle system responsible for urinary containment. Reeducation was the goal.
Working with patients individually, he had them practice contracting their pubococcygeal muscles until a perineometric response was elicited. Once they learned how to trigger perineometric response, he prescribed 3 bouts a day of 100 contractions each with the perineometer to build muscle strength. Once off to a good start, he suggested adding another 150 a day without the perineometer. Each contraction was held for several seconds and interspersed with a relaxation period.
Kegel reported complete relief in 6 to 8 weeks if the problem was of the simple type; 75% of his 500 cases were of the simple type. In 1956, he reported that 86% of 455 patients undergoing training at his perineometer clinic were cured by physiologic, nonoperative therapy. Age was irrelevant provided cognitive function was present. He also noted that 50% of these women had had a previous pelvis repair operation.2
FIG.5. Pelvic muscle strength vs. age.EMG-measured muscle strength (in microvolts) is plotted on the vertical axis against the age of each of 100 subjects. Mean strength drops after age 50
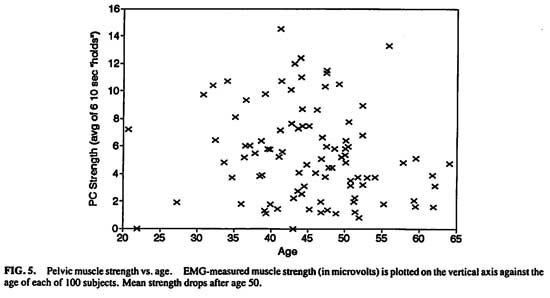
FIG.6. Nocturia among well women and age. The horizontal axis plots the age of 60 women against the vertical axis showing the number of times each woman gets up to urinate in the night. Nocturia becomes increasingly common after age 30
In spite of this positive launch, reported in JAMA in the 1950s, surgeons continued to perform surgery to “repair” S.U.I. with limited success.21 Biofeedback was tedious and the Kegel perineometer was not commercially available. By the late 1980’s with the development of an EMG perineometer, and commercial availability17 a series of research papers, often written by nurses, began to appear. These reports confirmed that pelvic muscle training was effective in reversing S.U.I. under a variety of conditions.3-12
Variations in training methods appear to account for variations in success. Women were most successful in overcoming S.U.I. when they had objective biofeedback using the score that was shown concurrent with their work of contracting their pelvic muscles. Failure to provide this biofeedback to women tended to produce ineffective results notwithstanding long periods of physician or a nurse time invested training patients through digital manipulation.5,8 Although the number of contractions per day that will optimize results and minimize unnecessary work remains to be determined, Kegel’s quantity, which set the highest demands produced the highest success rates.
Further studies with perineometric training showed that the use of vaginal balloons or other resistance devices is irrelevant when pelvic muscle training with objective biofeedback is provided at least weekly for about six weeks.6 Although vaginally placed cones of varying weights have recently been studied for use in training women to create sufficient muscle tension to retain ever heavier ones, the success rate with their use is modest6,10,11,12 compared with the purchase price ($140) and the better results obtained with perineometric biofeedback.1,2,8,17 When resistance devices placed in the vagina were added to biofeedback, they did not cause any problems. They just did not add to the effective results.
In summary, because stress urinary incontinence appears to be so pervasive, is related to multiparity, is reflected in poor perineometric scores, and is corrected with perineometric feedback training, S.U.I. testing should be made routinely available. A simple questionnaire test would alert the practitioner to S.U.I.’s presence.
We recommend that clinicians and other investigators be especially alert to screening for S.U.I. in women who are multiparous or menopausal. The Agency for Health Care Policy and Research, in 1992, recommended guidelines for the treatment of all forms of incontinence including S.U.I. They called for the use of (1) behavioral methods, (2) drugs and, (3) surgery, in that order, for the treatment of S.U.I. When anatomical defects are not etiologically related to neurological procedures, and in the absence of obvious disease, such as Multiple Sclerosis, the patient should be referred to a biofeedback specialist for pelvic muscle training as a first step.
Physicians should educate patients about the potential cure through correctly executed pelvic-muscle-strengthening exercises. It appears possible that by retraining their pelvic muscles, women can regain their freedom from the inevitable discomfort associated with loss of control. We estimate that this routine health assessment would improve the quality of life for many women. Controlled studies comparing different forms of biofeedback by the same investigator would improve our understanding of the current options and their relative efficiencies.
References
1. Kegel AH. Physiologic therapy for urinary stress incontinence. JAMA 1951;46:915.
2. Kegel AH. Stress incontinence of urine in women: Physiologic treatment. J Int Col Surgeons 1956: April:487.
3. Brink C, Sampsell C, Wells T, Diokno A, Gillis G. A digital test for pelvic muscle strength in older women with urinary incontinence. Nursing Research 1989;38:196.
4. Henderson J, Taylor K. Age as a variable in an exercise program for the treatment of simple urinary stress incontinence. JOGNN 1987;266.
5. Wilson PD, Al Samarrai T, Deakin M, Kolbe E, Brown ADG. An objective assessment of physiotherapy for genuine female stress incontinence. Brit J Obstet & Gynecology 1987;94:575.
6. Ferguson K, McKey P, Bishop K, Kloen P, Verhuel J, Dougherty M. Urinary stress incontinence: The effect of pelvic muscle exercise. Obstetrics and Gynecology 1990;45:671.
7. Kuhns-Hastings J. Management of female incontinence. JAAOHN 1988;36:78.
8. Burgio KL, Robinson JC, Engel BT. The role of biofeedback in Kegel exercise training for urinary stress incontinence. Am J Obstet Gynecol 1986;154:58.
9. Sampselle CM. Changes in pelvic muscle strength and stress urinary incontinence associated with childbirth. J Obstet Gynecol & Neonatal Nursing 1990;19:371.
10. Peattie AB, Plevnick S, Stanton SL. Vaginal cones: A conservative method of treating genuine urinary stress incontinence. Brit J Obstet & Gynecol 1988:95:1049.
11. Wilson P Don, Borland M. Vaginal cones for the treatment of genuine stress incontinence. Aust NZ J Obstet Gynecol 1990;30:157.
12. Olah KS, Bridges N, Denning J, Farrar DJ. The conservative management of patients with symptoms of stress incontinence: A randomized prospective study comparing weighted vaginal cones and intereferential therapy. Am J Obstet & Gynecol 1990;162:87.
13. Cutler WB, Garcia CR, McCoy N. Perimenopausal sexuality. Arch Sex Beh 1987;16:225.
14. Cutler WB, McCoy N., Davidson JM. Sexual behavior, steroids and hot flashes are associated during the perimenopause. Neuroendo L 1983;5:185.
15. McCoy NL, Davidson JM. A longitudinal study of the effects of menopause on sexuality. Maturitas 1985;7:203.
16. Cutler WB, Garcia CR. Menopause: A Guide for Women and the Men who Love Them. WW Norton, 1992.
17. Perry J. and Talcott L. The role of home trainers in Kegel’s exercise program for the treatment of incontinence. Ostomy and Wound Management 1990;30:46.
18. Kuppermann HS, Wetchler BB, Blatt MGH. Contemporary theory of menopausal syndrome. JAMA 1959;171:1627.
19. Nygaard I, DeLancey JO, Arnsdorf L, Murphy E. Exercise and incontinence. Ob Gyn 1990;75:848.
20. Dougherty MC, Bishop KC, Mooney RA, Gimotty PA, Landy LB. Variation in intravaginal pressure measurements. Nursing Res 1991;40:282.
21. Chalker R, Whitmore KE. Overcoming Bladder Disorders. 1990 New York: Harper and Row, 1990.
Related Links:
Philadelphia Study on Athena Pheromone 10X for men
San Francisco State University study on Athena Pheromone 10:13
Boston Study on Postmenopausal women
About the Scientific Process of a double-blind study
Full Bibliography of Dr. Cutler's work
Menopause: A Guide for Women and Those Who Love Them
Hysterectomy: Before and After
More information on health over forty
More information on bone health as we age
More information on Dr. Cutler's pheromone discovery
Please visit our guide to the medical school admissions process for premeds: Searching For Admission: The Smart PreMed Student's Guide to Applying for Medical School
" My research has consistently focused on what behavior a woman can engage in to increase her power, well-being, and vitality."
---Winnifred B. Cutler, Ph.D.
A portion of the profits from our book and pheromone sales helps to fund Athena's on-going research.